- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Immunotherapy in the Frontline Management of Advanced and Metastatic NSCLC
The frontline treatment paradigm for patients with advanced or metastatic non−small cell lung cancer (NSCLC) has changed dramatically in the past decade amid efforts to tackle this leading cause of cancer-related mortality. Immune checkpoint inhibitors (ICIs) targeting the programmed cell death protein 1 (PD-1) receptor and its ligand PD-L1 are an important therapeutic option for patients whose tumors lack genetic alterations that dictate response to molecularly targeted therapies. With a growing number of FDA-approved ICI monotherapy and combination therapy options for first-line therapy, the use of biomarkers such as PD-L1 expression has become increasingly important in guiding therapeutic decision making. Presently, PD-L1 expression remains a key biomarker in this setting, in spite of its limitations. This article will evaluate the current and evolving clinical trends in the use of ICIs in the frontline management of metastatic NSCLC, as well as the challenges associated with PD-L1 expression analysis and biomarker implementation.
Am J Manag Care. 2021;27(suppl 18):S323-S332. https://doi.org/10.37765/ajmc.2021.88769
The Burden of Lung Cancer
Lung cancer is one of the most common types of cancer and a leading cause of cancer-related death among both sexes in the United States, with 235,760 new cases and 131,880 deaths due to lung cancer anticipated in 2021.1 Although histologically heterogeneous, it can be broadly classified into non−small cell lung cancers (NSCLCs), which make up approximately 85% of cases, and small cell lung cancers. NSCLC is further divided into adenocarcinomas, which account for approximately 40% of cases, squamous cell carcinomas, representing 25% to 30% of cases, and large cell carcinomas comprising 5% to 10%.2
Lung cancer is more commonly diagnosed in older patients; the incidence in individuals under age 40 is low, then gradually increases to a peak between 65 and 84 years of age, with a median age at diagnosis of 71 years. In the United States, the incidence of lung cancer and associated mortality rates is highest among Black men and women compared with other racial and ethnic groups. Hispanic individuals have lower lung cancer incidence than non-Hispanics and the lowest annual incidence is observed among Asians/Pacific Islanders.2-4
The most significant risk factor for lung cancer is inhalation of carcinogens from cigarette smoking; more than 80% of lung cancer-related deaths are attributed to smoking, with an additional approximately 3% resulting from secondhand smoke exposure.1,5 However, nonsmoking-related lung cancer represents a significant and growing burden, particularly among women and patients younger than 55 years.1,6 A number of other risk factors are associated with the development of lung cancer, most notably exposure to radon.7
The United States Preventive Services Task Force (USPSTF) recommends annual screening with a chest low-dose computed tomography (CT) scan in high-risk individuals (30 pack-year smoking history) between the ages of 55 and 80 years, unless the individual has not smoked for 15 years or has a limited life span, as this is associated with reduced mortality.8,9 Owing to the implementation of screening guidelines, in addition to efforts to reduce cigarette smoking rates and advancements in treatment, the incidence of lung cancer has been declining since the 1980s among men and since the later part of the 2000s among women.2
Exploiting the Antitumor Immune Response
For patients who present with early-stage lung cancer, surgical resection remains the optimal treatment. However, less than 20% of cases are diagnosed at this stage; the majority are diagnosed at an advanced stage with distant metastasis and 5-year survival is just 6%.10 Historically, the mainstay of treatment for these patients was platinum-based doublet chemotherapy; however, a greater understanding of the molecular pathogenesis of NSCLC facilitated the development of various targeted therapies that have improved survival rates for patients with certain subtypes of metastatic NSCLC.11
For patients whose tumors lack mutations that respond to molecularly targeted therapies, the treatment paradigm has also changed significantly in the past decade with the development of immune checkpoint inhibitors (ICIs), which capitalizes on the potential of the immune response to restrain tumor growth.11 Although cancers derive from a patient’s own tissues, they are characterized by genetic abnormalities that can mark the cancer cells as nonself, triggering an antitumor immune response. A number of steps are required for the antitumor immune response to result in effective killing of cancer cells, described as the cancer-immunity cycle. The continual evolution of cancer cells, however, often allows them to develop ways of evading or actively suppressing the antitumor immune response by disrupting various points in the cancer-immunity cycle.12
The premise of immunotherapy is to boost or reinstate antitumor immunity. To date, the focus has been on the T cells as the major cytotoxic effectors of the immune system. T-cell activity is tightly regulated by the integration of multiple signals to ensure self-tolerance and prevent autoimmunity. Following the primary signal generated by interaction between the T-cell receptor and the antigen-MHC complex on the surface of an antigen-presenting cell, a secondary signal is generated by the binding of inhibitory or stimulatory receptors with their corresponding ligands. These so-called immune checkpoints determine whether the T cell is switched on (stimulatory) or off (inhibitory). Programmed cell death protein 1 (PD-1) receptor is the best-known inhibitory receptor and cancer cells often exploit its immunosuppressive function by expressing one of its ligands, PD-L1, on their surface or by recruiting PD-L1−expressing immune cells to the tumor microenvironment, to downregulate T cell cytotoxic activity and prevent the host from mounting an effective antitumor immune response. ICIs, monoclonal antibodies designed to block the activity of PD-1 or its ligands, have elicited durable responses across a range of solid tumors.13,14
Uptake of PD-L1 Testing
PD-L1 testing via immunohistochemical analysis has become an important tool for clinical decision making in patients with advanced or metastatic NSCLC. Several assays have been developed that differ in the specific antibodies used to detect PD-L1 expression, the detection systems employed, and the thresholds for PD-L1 positivity (Table 115). Different ICIs were approved together with different companion diagnostics; pembrolizumab and cemiplimab were approved in conjunction with the PD-L1 IHC 22C3 pharmDx assay, while the Ventana PD-L1 (SP142) assay is approved to identify patients eligible for atezolizumab treatment.16-18 The 22C3 assay measures the tumor proportion score (TPS), which is defined as the total number of tumor cells demonstrating partial or complete membrane staining of any intensity. The SP142 assay reports a tumor cell and immune cell score, measuring the number of tumor cells that stain positive for PD-L1 as a proportion of the total number of tumor cells and the percentage of the total tumoral area occupied by positive immune cells, respectively.19
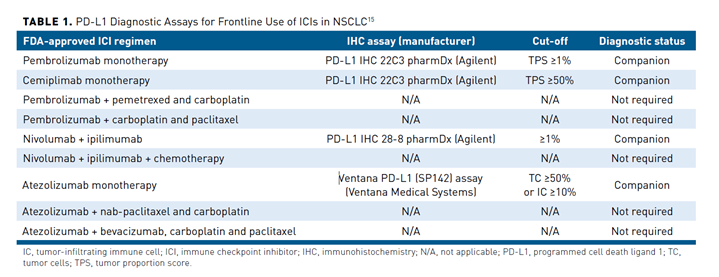
Although testing for PD-L1 expression is recommended as routine clinical practice prior to first-line therapy, it is not without challenges. Among the most significant is the lack of standardization among the currently available tests. Although studies evaluating assay concordance have been promising, interchangeable use of assays and cutoffs could lead to misclassification of PD-L1 status for some patients. Notably, several analyses have found that the SP142 assay consistently labels fewer tumor cells than other PD-L1 assays.20,21 The interpretation of PD-L1 IHC assays is challenging because of the different methods and thresholds used for determining positive results. A number of studies have demonstrated substantial levels of variation between pathologists performing the interpretation using FDA-approved PD-L1 tests.22 Additionally, PD-L1 testing does not provide the same clear binary results as other biomarker tests used in the management of lung cancer. Instead, there is significant inter- and intratumor heterogeneity in PD-L1 expression levels, as well as varying PD-L1 expression levels over time, which can also be impacted by previous and concurrent treatments. Due to this heterogeneity, a patient’s PD-L1 expression status can be significantly impacted by the sampling method. Studies have demonstrated discordance in PD-L1 expression levels measured in biopsy and surgically resected specimens; however, the latter may not be available and a single biopsy is unlikely to capture heterogeneity.23 Not all patients with PD-L1 expression respond and responses can be seen in patients who are PD-L1 negative, further highlighting the imperfect nature of PD-L1 expression as a predictive biomarker and that there are additional factors that influence ICI efficacy, which are only beginning to be elucidated.24 These challenges can impact the uptake and appropriate use and interpretation of PD-L1 testing.
Alternative biomarkers, such as tumor mutational burden, defined as the number of mutations per megabase of DNA, are being explored in the setting of NSCLC; however, despite its limitations, PD-L1 IHC testing continues to be the recommended predictive biomarker at the current time.
Expanding Frontline Options
Initially approved for patients who had progressed on standard chemotherapy, a growing number of ICI options are now available for frontline management of patients with advanced or metastatic NSCLC who do not have any targetable oncogene mutations.
ICI Monotherapy
Pembrolizumab (a PD-1-targeted monoclonal antibody), atezolizumab (a PD-L1-targeted antibody), and most recently, cemiplimab (PD-1), are each approved by the FDA as single agents for first-line treatment of patients with advanced NSCLC with high levels of PD-L1 expression (TPS ≥50%), as determined by an FDA-approved test. Pembrolizumab approval in this setting was based on the KEYNOTE-024 trial, while IMpower110 was the pivotal trial of atezolizumab monotherapy in previously untreated patients with advanced NSCLC with PD-L1 expression on at least 1% of tumor cells or immune cells within the tumoral area.25-27 In February 2021, cemiplimab became the third ICI to receive FDA approval for first-line treatment of patients with advanced NSCLC as a single agent on the basis of the EMPOWER-Lung 1 study.28 In each of these studies, ICI monotherapy resulted in a statistically significant overall survival (OS) benefit compared with platinum-based chemotherapy in patients who had a PD-L1 expression ≥50%, while also yielding significant improvements in other key clinical end points (Table 226-30).
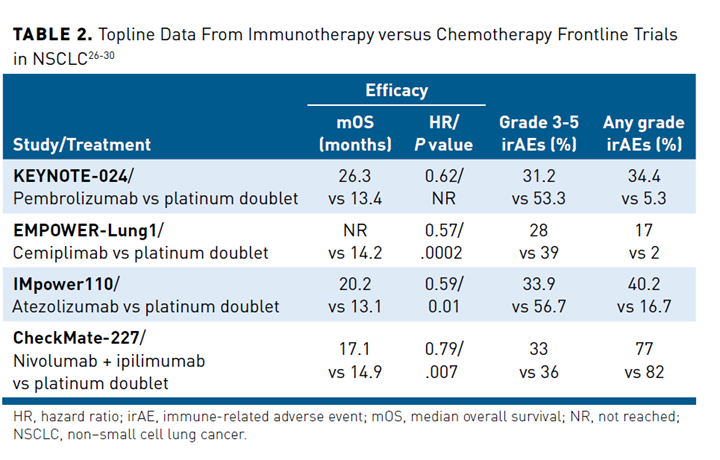
Pembrolizumab is also approved as monotherapy for previously untreated patients with metastatic NSCLC whose tumors express lower levels of PD-L1 (≥1%) based on the results of the KEYNOTE-042 trial, in which OS was significantly longer in patients treated with pembrolizumab compared with chemotherapy among patients with detectable PD-L1 expression in 1% or more of tumor cells (16.7 months vs 12.1 months, respectively; HR, 0.81; P = .0018).31
Combination Therapy
Despite these successes, only a fraction of patients respond to monotherapy.32,33 Efficacy is limited by a number of factors, including the potential for tumors to evade or suppress the resulting antitumor immune response. Combination therapies have been explored as a way to broaden the scope of ICI therapy. Chemoimmunotherapy approaches demonstrated encouraging results in clinical trials and several such combinations are now approved by the FDA for treatment of advanced NSCLC in the first-line setting, with approvals based on the demonstration of improved OS for each combination regimen compared with chemotherapy alone
(Table 334-38). The molecular mechanisms underlying the synergy between chemotherapy and immunotherapy are not completely understood; however, at least in part it results from the immunomodulatory properties of chemotherapy.39
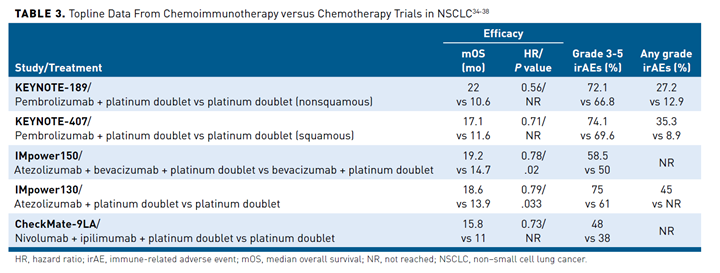
The combination of pembrolizumab and pemetrexed plus platinum-based chemotherapy is approved for the first-line treatment of metastatic nonsquamous NSCLC regardless of PD-L1 expression level based on the KEYNOTE-189 trial.34 The combination of pembrolizumab and a slightly different chemotherapy regimen (carboplatin plus paclitaxel or albumin-bound paclitaxel) is approved for the first-line treatment of metastatic squamous NSCLC regardless of PD-L1 expression based on data from the KEYNOTE-407 trial.35
Atezolizumab is approved in combination with chemotherapy, with or without the vascular endothelial growth factor (VEGF)-targeted monoclonal antibody bevacizumab, as first-line therapy for patients with metastatic nonsquamous NSCLC following improved outcomes in the IMpower150 and IMpower130 trials.36,37,40 Bevacizumab and other drugs that target VEGF and its receptors are anti-angiogenic agents designed to normalize the tumor vasculature, which is structurally and functionally abnormal. The rationale for combining anti-angiogenic drugs with ICIs is that this abnormal tumor vasculature may contribute to cancer immune evasion by preventing sufficient immune cell access to the tumor and its microenvironment.41,42 Notably, neither atezolizumab combination is currently approved for the treatment of squamous NSCLC. In the IMpower131 study, the combination of nab-paclitaxel, carboplatin, and atezolizumab improved progression-free survival (PFS) but not OS in this NSCLC subtype.43
In May 2020, the FDA approved the combination of nivolumab and ipilimumab for the treatment of metastatic squamous or nonsquamous NSCLC in the first-line setting for patients with PD-L1 expression levels of 1% or more. Ipilimumab and nivolumab have complementary mechanisms of action, with ipilimumab blocking the activity of a different immune checkpoint receptor, cytotoxic T-lymphocyte antigen 4 (CTLA-4). In the pivotal CheckMate 227 trial, 29% of patients treated with combination therapy were still alive and more than one-third remained in response after 4 years of follow-up.44 Based on the phase 3 CheckMate 9LA study, the combination of nivolumab and ipilimumab plus 2 cycles of platinum-doublet chemotherapy is another frontline treatment option for patients with advanced NSCLC regardless of PD-L1 expression levels and histology.38,45
Recommended Treatment Paradigm
With the growing number of competing FDA-approved ICIs for the frontline management of advanced NSCLC, optimal treatment selection depends increasingly on patient-specific factors. The National Comprehensive Cancer Network (NCCN) has developed evidence-based guidelines to help guide treatment selection (Figure 111). Testing for actionable biomarkers, including PD-L1 expression, is recommended before first-line treatment in patients with metastatic NSCLC, if clinically feasible. Patients with actionable molecular variants in driver oncogenes (eg, epidermal growth factor receptor and anaplastic lymphoma kinase should receive first-line targeted therapy for that oncogene, independent of PD-L1 expression levels. For patients without these targetable alterations, the choice of therapy is guided by PD-L1 expression level and histology.11
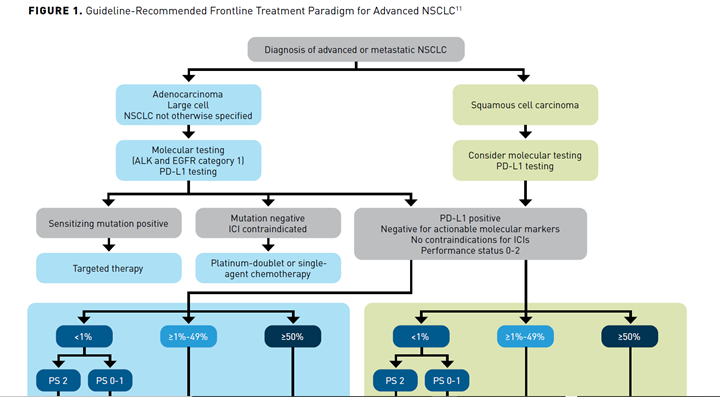
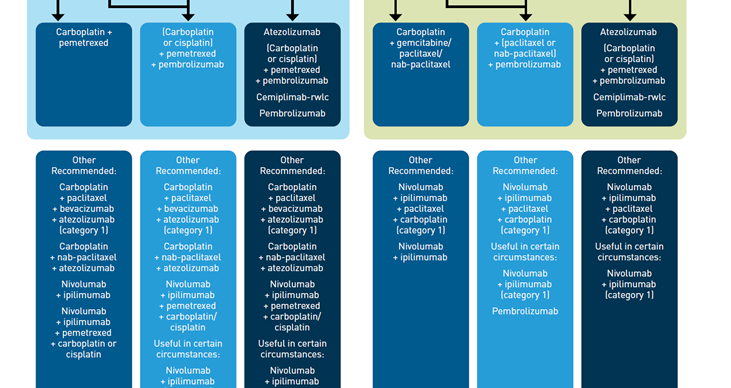

PD-L1 greater than or equal to 50%
Regardless of histology, single-agent therapy with pembrolizumab, atezolizumab, or cemiplimab are preferred treatment options for patients with metastatic NSCLC with no contraindications to ICIs, negative test results for actionable molecular alterations, and a performance status (PS) of 0-2.11
PD-L1 greater than or equal to 1% to 49%
For patients with lower levels of PD-L1 expression, pembrolizumab and chemotherapy combination therapy is preferred over monotherapy, based on currently available clinical data. For patients with nonsquamous NSCLC, the preferred chemotherapeutic combination is pemetrexed and either carboplatin or cisplatin, while for squamous NSCLC, a regimen of carboplatin and either paclitaxel or albumin-bound paclitaxel is preferred.
The NCCN also recommends single-agent pembrolizumab as a category 2A treatment option that may be useful in certain circumstances for patients with lower PD-L1 expression levels, for example, in patients who cannot tolerate platinum-based chemotherapy.11
Independent of PD-L1 Status
The combination of pembrolizumab and chemotherapy is a preferred frontline treatment option regardless of PD-L1 expression level and offers an equally effective treatment option to monotherapy in patients with high levels of PD-L1 expression. It may offer an advantage in patients with certain clinical factors, such as rapidly progressing or very extensive disease.
The combination of atezolizumab, bevacizumab, and paclitaxel is a category 1 “other recommended” option for the frontline treatment of patients with metastatic nonsquamous NSCLC, regardless of PD-L1 expression levels, but is not currently approved for patients with squamous NSCLC. Likewise, atezolizumab in combination with albumin-bound paclitaxel, without the addition of bevacizumab, is also an “other recommended” option in this setting, but is a category 2A recommendation.
Irrespective of histology and PD-L1 expression level, nivolumab plus ipilimumab in combination with chemotherapy is a category 1 other recommended treatment option, with choice of chemotherapy determined by histology. The dual nivolumab and ipilimumab offers a chemotherapy-sparing alternative regimen, which may be useful in certain circumstances.11
PD-L1 less than 1%
For patients with PD-L1 expression levels less than 1%, the recommended treatment options depend on their PS. Chemotherapy remains the standard for patients with a PS of 2, while for patients with PS 0-1 the recommended treatment paradigm is the same as for patients with PD-L1 expression levels of greater than or equal to 1% to 49%, with pembrolizumab in combination with chemotherapy the preferred choice, while atezolizumab plus chemotherapy, with or without bevacizumab (with bevacizumab is a category 1 recommendation), and nivolumab plus ipilimumab with or without chemotherapy (the latter being the category 1 recommendation) are other recommended options.11
Emerging Options
In addition to approved immunotherapy options, there are a number of emerging investigational strategies for frontline management of advanced NSCLC.
ICI plus Chemotherapy
In the phase 3 EMPOWER-Lung 3 trial, the combination of cemiplimab and platinum-based chemotherapy was evaluated as first-line treatment of advanced/metastatic NSCLC. The trial was halted early due to a statistically significant improvement in OS in the combination arm at the interim analysis (mOS: 22 months vs 13 months; HR, 0.71; P = .014). No new safety signals were reported.46
Tislelizumab is a novel PD-1-targeted antibody designed to have reduced binding to Fc-gamma receptors, in an effort to overcome a potential mechanism of resistance to PD-1 inhibitor antibody therapy.47,48 Several ongoing phase 3 clinical trials are evaluating the efficacy and safety of tislelizumab in combination with chemotherapy. In the RATIONALE-304 trial, the combination of tislelizumab plus pemetrexed and platinum-based chemotherapy is being evaluated in patients with advanced nonsquamous NSCLC, while the RATIONALE-307 trial is testing the addition of tislelizumab to paclitaxel or nab-paclitaxel plus carboplatin in advanced squamous NSCLC. In both trials, the addition of tislelizumab to chemotherapy significantly improved PFS, ORR, and duration of response compared with chemotherapy alone.49,50 The combination is approved in China for squamous NSCLC and is under review for nonsquamous NSCLC.51,52
ICI + Targeted Therapy
ICIs also are being explored in combination with targeted therapies in a number of phase 3 clinical trials, based on preclinical studies that suggest potential synergistic activity. Notably, the LEAP clinical program is evaluating the combination of pembrolizumab and the multikinase inhibitor lenvatinib across a range of solid tumor types. In the LEAP-006 trial, the combination of pembrolizumab, platinum-based chemotherapy, and lenvatinib demonstrated impressive response rates and acceptable safety and tolerability in the first part of this study in patients with advanced nonsquamous NSCLC.53 The phase 3 LEAP-007 trial is evaluating the combination of pembrolizumab and lenvatinib without chemotherapy in patients with metastatic NSCLC. Several ongoing studies are also assessing ICIs in combination with poly (ADP-ribose) polymerase inhibitor therapy in the frontline setting.
Dual Immunotherapy Combinations
Among other notable emerging treatment options are several dual immunotherapy combinations. In the phase 2 CITYSCAPE trial, the combination of atezolizumab and tiragolumab (an inhibitor of an alternative inhibitory checkpoint protein TIGIT, which is expressed on multiple immune cells, including T cells) demonstrated clinically meaningful improvements in ORR and PFS over a median follow-up of 10.9 months.54 The combination was granted breakthrough therapy designation in January 2021 and the phase 3 SKYSCRAPER-01 trial of this combination is ongoing.55 Meanwhile, in the POSEIDON trial, the combination of durvalumab (PD-1 inhibitor), tremelimumab (CTLA-4 inhibitor), and chemotherapy demonstrated a statistically significant OS benefit compared with chemotherapy alone.56
Immune-related Adverse Events (irAEs)
Immune checkpoint blockade results in activation of T cells against tumor cells, which underlies their antitumor efficacy but can also result in unique irAEs. irAEs can impact virtually any organ, with varying frequency and severity across patients and according to the specific ICI (Figure 257). CTLA-4 inhibitors are associated with more frequent and severe irAEs than PD-1 and PD-L1 inhibitors, while PD-1 inhibitors tend to elicit a higher rate of irAEs compared with PD-L1 inhibitors. A patient’s overall health, the presence of comorbidities, or receipt of prior ICI therapy can impact the risk of irAEs.58-60
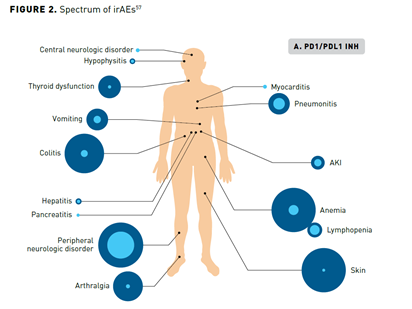
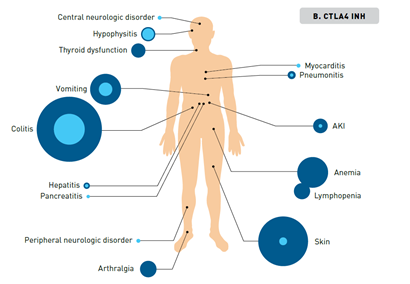
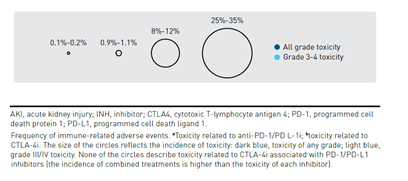
In general, irAEs are mild or moderate in severity and are reversible with appropriate timely management, but some can become severe or fatal if not promptly diagnosed and appropriately managed. Common high-grade AEs include diarrhea and severe skin reactions, while cardiac, hematologic, and neurologic irAEs are rarer causes of potentially severe toxicity. A notable exception to the reversibility of irAEs are those that affect the endocrine system. The pituitary, thyroid, and adrenal glands are the most commonly affected endocrine organs, but inflammation of individual glands also leads to dysfunction in downstream target organs, resulting in a diverse range of symptoms that can be relatively nonspecific, making diagnosis challenging.59,60 An irAE of particular relevance to patients with lung cancer is pneumonitis, which although uncommon is potentially life-threatening and has been shown to occur more frequently and with an earlier onset in patients with NSCLC than those with other cancer types. The incidence of pneumonitis is higher in former or current smokers and in patients treated with combination therapy.61-63
The greater efficacy of combination therapy comes at the expense of increased toxicity. In particular, dual ICI combinations can lead to more frequent or severe irAEs. The combination of ICIs with chemotherapy or targeted therapies typically has less impact on the frequency and severity of irAEs because the toxicity profiles of the drugs are largely non-overlapping. However, these combinations can present a challenge in distinguishing irAEs from toxicities caused by the partner drug, which can have distinct underlying causes that may necessitate different management strategies.59,60
A distinguishing factor of irAEs is their time of onset (typically 3-6 months after treatment initiation) and longer duration compared with targeted therapies (Figure 364). In some cases, irAEs may develop months or even years after ICI treatment discontinuation.33,65 irAEs from CTLA-4 inhibitors tend to present sooner than those from PD-1 or PD-L1 inhibitors, and irAEs from combination therapies present sooner than those from monotherapies.60
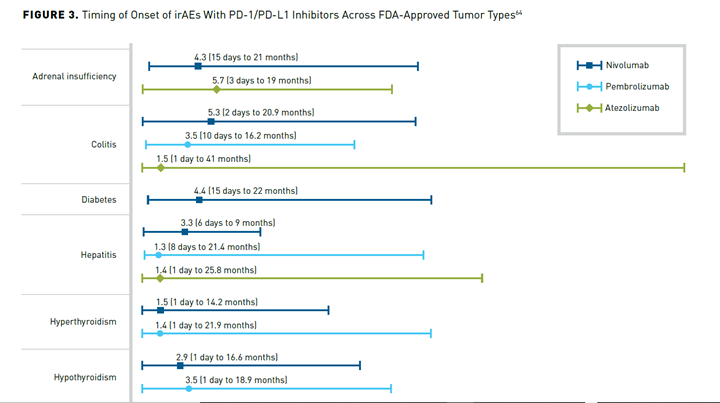
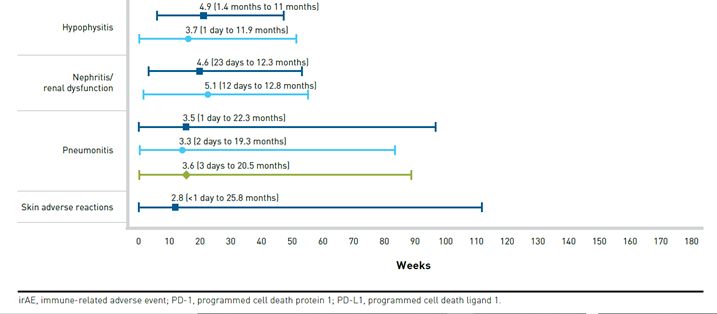
There is some evidence that patients who experience certain irAEs may experience better treatment outcomes. While further investigation is still needed, this could have implications for retreatment with ICIs after discontinuation due to irAEs.63,66,67 Furthermore, it could impact adherence if patients become concerned that they are not responding in the absence of irAEs.
Management of irAEs varies depending on the grade and type of irAE.33 Some can be managed with over-the-counter treatments and mild irAEs may not necessitate discontinuation while supportive care is administered. Immunosuppressants, predominantly corticosteroids, are the primary treatment used in higher-grade irAEs, and short-term use of corticosteroids does not appear to reduce antitumor efficacy of ICIs. irAEs that do not respond to corticosteroids may require additional treatment with other immunomodulatory drugs.67 For moderate/severe irAEs, temporary or permanent discontinuation of therapy is recommended. Re-administration of PD-1 or PD-L1 inhibitors after high-grade irAEs has been shown to be well tolerated; the risk of recurrence must be weighed against the potential benefits of reinitiating therapy. Irreversible endocrinopathies are often managed with endocrine-directed therapies and ICIs can be resumed in stable patients.59,60 Given the broad spectrum of irAEs and their unique nature, their effective management requires a coordinated multidisciplinary team of specialists.68
Conclusions
Immunotherapy has shaped a new standard of care for patients with advanced or metastatic NSCLC who lack targetable molecular alterations. The preferred treatment paradigm continues to evolve as new ICI-based regimens are approved and with growing appreciation of the importance of PD-L1 expression status in guiding frontline therapeutic choices. With a number of ongoing studies reporting promising data for ICI-chemotherapy, ICI-targeted therapy, and dual immunotherapy combinations, the stage is set for the treatment of metastatic NSCLC to further evolve over the next few years. Although PD-L1 expression is an established predictive biomarker, it is not without limitations that may drive the implementation of alternative biomarkers that further help to shape frontline treatment to ensure optimal outcomes for patients with a tumor type that remains a leading cause of cancer-related mortality.
Author affiliation: Eve M Segal, PharmD, BCOP,islead clinical pharmacist, Hematology/Oncology, University of Washington Medical Center/Seattle Cancer Care Alliance, Seattle, WA.
Funding source: This activity is supported by educational grants from BeiGene, Ltd. and Merck Sharp & Dohme Corp.
Author disclosure: Dr Segal has no relevant financial relationships with commercial interests to disclose.
Author information: Concept and design; drafting of the manuscript; and critical revision of the manuscript for important intellectual content.
Address correspondence to: segaleve@seattlecca.org
Medical writing and editorial support provided by: Jane de Lartigue, PhD
REFERENCES
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi:10.3322/caac.21654
2. Duma N, Santana-Davila R, Molina JR. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623-1640. doi:10.1016/j.mayocp.2019.01.013
3. American Lung Association. Lung Cancer Fact Sheet. Accessed August 8, 2021. www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet
4. Schabath MB, Cress WD, Muñoz-Antonia T. Racial and ethnic differences in the epidemiology of lung cancer and the lung cancer genome. Cancer Control. 2016;23(4):338-346.
5. Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31-54. doi:10.3322/caac.21440
6. Jeon J, Holford TR, Levy DT, et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med. 2018;169(10):684-693. doi:10.7326/M18-1250
7. Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol. 2012;10(3):157-164.
8. Moyer VA, US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
9. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
10. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. Accessed August 3, 2021. https://seer.cancer.gov/statfacts/html/lungb.html.
11. National Comprehensive Cancer Network. NCCN Guidelines, Non-Small Cell Lung Cancer, v5.2021. June 15, 2021. Accessed August 8, 2021. www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
12. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
13. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29-39. doi:10.1016/j.intimp.2018.06.001
14. Nandi D, Pathak S, Verma T, et al. T cell costimulation, checkpoint inhibitors and anti-tumor therapy. J Biosci. 2020;45:50.
15. US Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Updated August 4, 2021. Accessed August 30, 2021. www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools
16. Agilent Technologies Inc. Press release. Agilent PD-L1 IHC 22C3 pharmDx receives expanded FDA approval in non-small cell lung cancer (NSCLC). February 22, 2021. Accessed August 3, 2021. www.agilent.com/about/newsroom/presrel/2021/22feb-ca21004.html
17. Agilent Technologies Inc. PD-L1 IHC 22C3 pharmDx Testing for NSCLC. Accessed August 3, 2021. www.agilent.com/en-us/products/pharmdx/pd-l1-ihc-22c3-pharmdx-testing-for-nsclc
18. US Food and Drug Administration. FDA approves atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression. May 18, 2020. Accessed August 3, 2021. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-first-line-treatment-metastatic-nsclc-high-pd-l1-expression
19. Arora S, Velichinskii R, Lesh RW, et al. Existing and emerging biomarkers for immune checkpoint immunotherapy in solid tumors. Adv Ther. 2019;36(10):2638-2678. doi:10.1007/s12325-019-01051-z
20. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208-222. doi:10.1016/j.jtho.2016.11.2228
21. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint Phase 2 Project. J Thorac Oncol. 2018;13(9):1302-1311. doi:10.1016/j.jtho.2018.05.013
22. Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345-362. doi:10.1038/s41571-021-00473-5
23. Wang M, Wang S, Trapani JA, Neeson PJ. Challenges of PD-L1 testing in non-small cell lung cancer and beyond. J Thorac Dis. 2020;12(8):4541-4548. doi:10.21037/jtd-2019-itm-010
24. Kim H, Chung J-H. PD-L1 testing in non-small cell lung cancer: past, present, and future. J Pathol Transl Med. 2019;53(4):199-206. doi:10.4132/jptm.2019.04.24
25. Davda J, Declerck P, Hu-Lieskovan S, et al. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J ImmunoTher Cancer. 2019;7(1):105. doi:10.1186/s40425-019-0586-0
26. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. 2021;39(21):2339-2349. doi:10.1200/JCO.21.00174
27. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328-1339. doi:10.1056/NEJMoa1917346
28. Sezer A, Kilickap S, Gümüs¸ M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592-604. doi:10.1016/S0140-6736(21)00228-2
29. Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: three-year update from CheckMate 227 Part 1. J Clin Oncol. 2020;38(15_suppl):9500-9500. doi:10.1200/JCO.2020.38.15_suppl.9500
30. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
31. Mok TS, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated,
PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042):
a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830.
doi:10.1016/S0140-6736(18)32409-7
32. Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med. 2019;6:119. doi:10.3389/fmed.2019.00119
33. Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs. 2019;6(2):154-160. doi:10.4103/apjon.apjon_3_19
34. Rodriguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881-895.doi:10.1016/j.annonc.2021.04.008
35. Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657-1669. doi:10.1016/j.jtho.2020.06.015
36. Socinski MA, Mok TS, Nishio M, et al. Abstract CT216: IMpower150 final analysis: Efficacy of atezolizumab (atezo) + bevacizumab (bev) and chemotherapy in first-line (1L) metastatic nonsquamous (nsq) non-small cell lung cancer (NSCLC) across key subgroups. Cancer Res. 2020;80(16 suppl):CT216-CT216. doi:10.1158/1538-7445.AM2020-CT216
37. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi:10.1016/S1470-2045(19)30167
38. Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. doi:10.1016/S1470-2045(20)30641-0
39. Leonetti A, Wever B, Mazzaschi G, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Updat. 2019;46:100644. doi:10.1016/j.drup.2019.100644
40. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi:10.1056/NEJMoa1716948
41. Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561-17566. doi:10.1073/pnas.1215397109
42. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52(9):1475-1485. doi:10.1038/s12276-020-00500-y
43. Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351-1360. doi:10.1016/j.jtho.2020.03.028
44. Paz-Ares L, Ciuleanu T-E, Lee J-S, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line therapy (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol. 2021;39(15_suppl):9016. doi:10.1200/JCO.2021.39.15_suppl.9016
45. Reck M, Ciuleanu T-E, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): two-year update from CheckMate 9LA. J Clin Oncol. 2021;39(15_suppl):9000. doi:10.1200/JCO.2021.39.15_suppl.9000
46. Phase 3 trial of Libtayo (cemiplimab-rwlc) combined with chemotherapy stopped early due to significant improvement in overall survival in patients with first-line advanced non-small cell lung cancer. News release. Regeneron. August 5, 2021. Accessed August 30, 2021. https://investor.regeneron.com/news-releases/news-release-details/phase-3-trial-libtayor-cemiplimab-rwlc-combined-chemotherapy
47. Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079-1090. doi:10.1007/s00262-018-2160-x
48. Liu S-Y, Wu Y-L. Tislelizumab: an investigational anti-PD-1 antibody for the treatment of advanced non-small cell lung cancer (NSCLC). Exp Opin Invest Drugs. 2020;29(12):1355-1364. doi:10.1080/13543784.2020.1833857
49. Wang J, Lu S, Yu X, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709-717. doi:10.1001/jamaoncol.2021.0366
50. Lu S, Wang J, Yu Y, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512-1522. doi:10.1016/j.jtho.2021.05.005
51. BeiGene announces acceptance of a supplemental new drug application for tislelizumab in combination with chemotherapy in first-line advanced non-squamous non-small cell lung cancer in China. News release. Globenewswire. June 19, 2020. Accessed August 4, 2021. www.globenewswire.com/en/news-release/2020/06/19/2050516/0/en/BeiGene-Announces-Acceptance-of-a-Supplemental-New-Drug-Application-for-Tislelizumab-in-Combination-with-Chemotherapy-in-First-Line-Advanced-Non-Squamous-Non-Small-Cell-Lung-Cancer.html
52. Businesswire. China National Medical Products Administration approves tislelizumab in combination with chemotherapy in first-line advanced squamous non-small cell lung cancer. January 13, 2021. Accessed August 4, 2021. www.businesswire.com/news/home/20210113005974/en/China-National-Medical-Products-Administration-Approves-Tislelizumab-in-Combination-with-Chemotherapy-in-First-Line-Advanced-Squamous-Non-Small-Cell-Lung-Cancer
53. Nishio M, Peled N, Zer A, et al. 1313P Phase III LEAP-006 safety run-in (Part 1): 1L pembrolizumab (Pembro) + chemotherapy (Chemo) with lenvatinib (Len) for metastatic NSCLC. Ann Oncol. 2020;31:S848-S849. doi:10.1016/j.annonc.2020.08.1627
54. Rodriguez-Abreu D, Johnson ML, Hussein MA, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol. 2020;38(15_suppl):9503-9503. doi:10.1200/JCO.2020.38.15_suppl.9503
55. Roche’s novel anti-TIGIT tiragolumab granted FDA breakthrough therapy designation in combination with Tecentriq for PD-L1-high non-small cell lung cancer. News release. Roche. January 5, 2021. Accessed August 4, 2021. www.roche.com/media/releases/med-cor-2021-01-05.htm
56. Imfinzi and tremelimumab with chemotherapy demonstrated overall survival benefit in POSEIDON trial for 1st-line stage IV non-small cell lung cancer. News release. AstraZeneca. May 7, 2021. Accessed August 9, 2021. www.astrazeneca.com/media-centre/press-releases/2021/imfinzi-and-tremelimumab-showed-survival-in-poseidon.html
57. Lemiale V, Meert A-P, Vincent F, et al. Severe toxicity from checkpoint protein inhibitors: what intensive care physicians need to know? Ann Intensive Care. 2019;9(1):25. doi:10.1186/s13613-019-0487-x
58. Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017;58:70-76. doi:10.1016/j.ctrv.2017.06.002
59. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563-580. doi:10.1038/s41571-019-0218-0
60. Medina P, Jeffers KD, Trinh VA, Harvey RD. The role of pharmacists in managing adverse events related to immune checkpoint inhibitor therapy. J Pharm Pract. 2020;33(3):338-349. doi:10.1177/0897190019885230
61. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616. doi:10.1001/jamaoncol.2016.2453
62. Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051-6060. doi:10.1158/1078-0432.CCR-16-1320
63. Remon J, Mezquita L, Corral J, Vilariño N, Reguart N. Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients. J Thorac Dis. 2018;10(suppl 13):S1516-S1533. doi:10.21037/jtd.2017.12.52
64. Daniels GA, Guerrera AD, Katz D, Viets-Upchurch J. Challenge of immune-mediated adverse reactions in the emergency department. Emerg Med J. 2019;36(6):369-377. doi:10.1136/emermed-2018-208206
65. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z
66. Remon J, Reguart N, Auclin E, Besse B. Immune-related adverse events and outcomes in patients with advanced non-small cell lung cancer: a predictive marker of efficacy? J Thorac Oncol. 2019;14(6):963-967. doi:10.1016/j.jtho.2019.02.031
67. National Comprehensive Cancer Network. NCCN Guidelines, Management of Immunotherapy-Related Toxicities, v3.2021. Accessed August 8, 2021. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
68. Londoño M-C, Reig M, RETOINMUNO Multidisciplinary Group. Multidisciplinary clinical approach to cancer patients with immune-related adverse events induced by checkpoint inhibitors. Cancers. 2020;12(11):E3446. doi:10.3390/cancers12113446
