- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Hyperlipidemia: Effective Disease Management With a Focus on PCSK9 Inhibitors
Abstract
Hyperlipidemia is a prevalent condition in the United States and a significant contributor to atherosclerotic cardiovascular disease (ASCVD). ASCVD is a primary cause of morbidity and mortality in the United States. Low-density lipoprotein cholesterol (LDL-C) is a causal factor for the development of ASCVD. Reductions in LDL-C produce a corresponding decrease in ASCVD risk for cardiovascular events. HMG-CoA reductase inhibitors, commonly referred to as statins, remain the gold standard of hyperlipidemia treatment. However, statin monotherapy is often ineffective in reducing LDL-C to treatment guideline-recommended levels, especially in high-risk patients with established ASCVD or familial hypercholesterolemia (FH). Statin therapy causes myalgias in 5% to 10% of patients, which may lead to inadequate dose optimization, nonadherence, or inability to take a statin. Clinical guidelines recommend add-on therapy with ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors when maximally tolerated statin therapy results in suboptimal LDL-C reduction. Hyperlipidemia, especially FH, is associated with substantial clinical and financial burden and is often undertreated. Although undertreatment is partially attributable to failure to optimize statin therapy, a significant portion of patients will require a PCSK9 inhibitor for adequate LDL-C reduction. Despite this, PCSK9 inhibitor utilization rates remain low. Barriers to treatment may include clinical inertia, high out-of-pocket costs, and pharmacy benefit access issues. Managed care pharmacists can help appropriate patients overcome these barriers to PCSK9 inhibitor use and improve the attainment of LDL-C goals and outcomes, especially in high-risk patients with FH or clinical ASCVD.
Am J Manag Care. 2021;27(3):S63-S69. https://doi.org/10.37765/ajmc.2021.88606
Introduction
Approximately one-third of all US deaths result from heart disease, stroke, or other cardiovascular disease (CV).1 Clinical atherosclerotic cardiovascular disease (ASCVD) includes a history of myocardial infarction (MI), acute coronary syndrome (ACS), peripheral artery disease (PAD), ischemic stroke (IS), transient ischemic attack, stable or unstable angina (UA), and coronary or other arterial revascularization.2 Despite improvements in ASCVD outcomes, ASCVD remains the primary cause of morbidity and death in the United States and worldwide, accounting for greater than $200 billion in estimated costs of medications, healthcare services, and lost productivity in the United States.3
Hyperlipidemia is a major risk factor for the development of ASCVD. Low-density lipoprotein cholesterol (LDL-C) is an atherogenic form of cholesterol whose level correlates with ASCVD risk and is the main target of lipid-lowering drug therapy. Effective strategies to better control hyperlipidemia may significantly impact prevention efforts and ultimately improve ASCVD morbidity and mortality rates.2
Epidemiology and Burden of Hyperlipidemia
Impact of Hyperlipidemia Within the United States
Prevalence
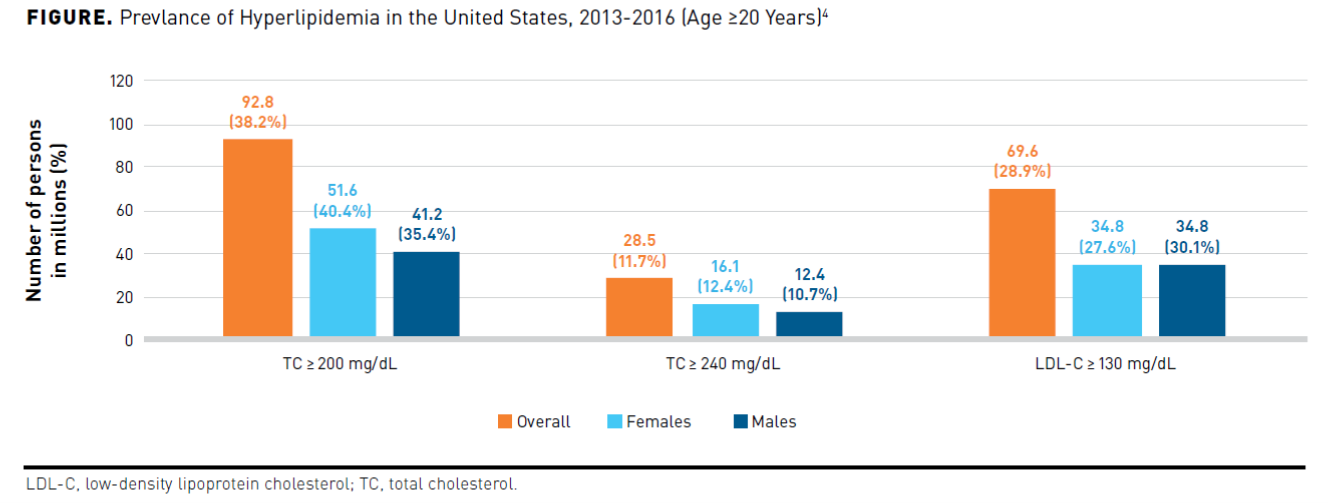
Approximately 38% of adults aged 20 years and older had a total serum cholesterol (TC) equal to or greater than 200 mg/dL and 12% of adults greater than 240 mg/dL, in the United States between 2013 and 2016. The prevalence of LDL-C equal to or greater than 130 mg/dL in adults 20 years and older was 28.9%, with a higher percentage in males (30.1%) than in females (27.6%). The Figure4 shows the prevalence of hyperlipidemia in the United States broken down by sex. Trends indicate the prevalence of LDL-C equal to or greater than 130 mg/dL in adults has decreased from 42.9% (1999-2000) to 29.4% (2015-2016). Greater use of cholesterol-lowering medications rather than dietary improvements may explain the decrease in mean cholesterol level. Prevalence rates, albeit improved, still indicate the presence of suboptimal control of hyperlipidemia.4
Clinical and Economic Burden of Hyperlipidemia in the United States
A retrospective cohort study using a US national longitudinal database examined the short-term and long-term clinical and economic burden associated with cardiovascular events (CVEs) in patients with hyperlipidemia. The study further stratified patients according to CV risk into low- and moderate-risk cohorts, history of a CVE cohort, and modified coronary heart disease (CHD) risk equivalent cohort. The study included 243,640 propensity-matched patients with and without new CVE to compare healthcare utilization and direct costs from 1 month through 3 years post-event. For patients with a new CVE, 65.8% had two or more new CVEs in the 3-year follow-up. All resource healthcare utilization was highest during the 1-month post-event in patients with hyperlipidemia with a new CVE and remained significantly higher at 2- and 3-year follow-up than those without a new CVE. The direct incremental healthcare costs in patients with hyperlipidemia with a new CVE ranged from $17,903 to $65,825, $474 to $19,617, and $2598 to $26,982, one year, 2 years, and 3 years post-CVE, respectively. The study demonstrated that patients with hyperlipidemia and a new CVE incurred significantly greater healthcare utilization and costs than those with hyperlipidemia without a new CVE up to 3 years post-event in all risk cohorts.5
ASCVD Risk Reduction
As suboptimal control has substantial clinical and financial burdens, improved hyperlipidemia management is necessary to reduce ASCVD risk. Evidence from prospective epidemiologic cohort studies, randomized controlled trials (RCTs), and Mendelian randomization studies support the LDL hypothesis. Excess LDL-C is a causal factor for the development of ASCVD.2,6,7 The Cholesterol Treatment Trialists’ Collaborators meta-analysis of 26 RCTs with varying intensities of statins demonstrated that each 38.6 mg/dL reduction in LDL-C reduced the rate of major vascular events (ie, composite of coronary death, MI, coronary revascularization, or stroke) by 22%, with no threshold identified in the LDL-C range studied.8
RCTs of nonstatin lipid-lowering agents also demonstrated the benefit of LDL-C reduction on CV outcomes. The IMPROVE-IT trial randomly assigned 18,144 patients stabilized after a recent ACS to receive either simvastatin 40 mg and placebo or ezetimibe 10 mg plus simvastatin 40 mg. Patients in the simvastatin monotherapy group achieved a mean LDL-C of 70 mg/dL, while those receiving simvastatin plus ezetimibe achieved a mean LDL of 54 mg/dL. IMPROVE-IT demonstrated that lowering LDL-C with the addition of ezetimibe produces an incremental reduction in CV event rate (HR, 0.936; 95 CI, 0.89-0.99; P = .016).9
More recently, CV outcomes trials using proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have demonstrated the effectiveness of even more aggressive LDL-C reductions in high-risk patients. In the randomized, double-blind, placebo-controlled ODYSSEY OUTCOMES trial, 18,924 patients with ACS (within the previous 1-12 months) treated with high-intensity or maximally tolerated statin therapy with an LDL-C of at least 70 mg/dL received either alirocumab 75 mg (9462 patients) or placebo (9642 patients) every 2 weeks, with a dose adjustment to 150 mg to target an LDL-C of 25-50 mg/dL. The median duration of follow-up was 2.8 years. The results demonstrated a 15% decrease in the primary composite end point (death from CHD), nonfatal MI, fatal or nonfatal IS, or UA requiring hospitalization) with alirocumab versus placebo (HR, 0.85; 95% CI, 0.78-0.93; P <.001). Those patients with a baseline LDL-C of 100 mg/dL or more experienced a greater absolute benefit from alirocumab concerning the primary composite end point than patients with a lower baseline level.10
A clinical study using the PCSK9 inhibitor evolocumab also demonstrated significant CV risk reduction. In the randomized, double-blind, placebo-controlled FOURIER trial, 27,254 patients with ASCVD and LDL-C of at least 70 mg/dL taking statin therapy received either evolocumab (140 mg every 2 weeks or 420 mg monthly) or placebo.11 Evolocumab significantly decreased the risk by 15% of theprimary composite end point (CV death, MI, hospitalization for UA, stroke, or coronary revascularization) versus placebo at 48 weeks (HR, 0.85; 95% CI, 0.70-0.92; P <.001). The evolocumab group achieved amedian LDL-C of 30 mg/dL. In summary, these 2 trials demonstrated that the PCSK9 inhibitors lowered LDL-C by approximately 60% and reduced the risk of major adverse cardiovascular events (MACE) by 15% in patients already receiving optimized statin therapy.10,11
The 2017 European Atherosclerosis Society (EAS) Consensus Panel statement examined current study evidence reaffirming the dose-dependent log-linear association between the absolute magnitude of exposure to elevated LDL-C and risk for ASCVD. Because LDL-C reductions have a causal and cumulative effect on ASCVD risk, the proportional risk reduction depends on the baseline LDL-C level, therapy duration, and the magnitude of LDL-C reduction. In addition to these other factors, the absolute risk reduction from LDL-C reduction also depends on baseline ASCVD risk. The lower the LDL-C level achieved through using statin and specific nonstatin therapies such as ezetimibe and PCSK9 inhibitors, the greater the clinical benefit realized. In particular, patients at high risk for ASCVD, especially patients with familial hypercholesterolemia (FH), may benefit from earlier decreases in LDL-C.6
FH
FH is an autosomal dominant genetic disorder resulting in hyperlipidemia that is often underdiagnosed and undertreated, with only 10% of the FH population diagnosed and receiving appropriate treatment.12,13 LDL-C is responsible for 75% of cholesterol transport in the body, and the majority of LDL is cleared from the plasma by LDL receptors (LDLR) on hepatic cellular membranes. The LDLR also binds and causes the cellular uptake of apolipoprotein B (apo B) and apolipoprotein E (apo E). Steroid regulatory element-binding proteins in the cell membrane regulate LDLR gene transcription through negative feedback. PCSK9 is a peptide responsible for LDLR recycling and lysosomal degradation in the hepatocyte. Mutations causing the absence of production of LDLRs or defective functioning of LDLRs can cause FH, as well as those affecting apo B binding and PCSK9 function.13 LDLR loss-of-function mutations, defective apo B receptor-binding, and PCSK9 gain-of-function mutations account for 80% to 90%, 5% to 10%, and less than 1% of all FH cases, respectively.14 Abnormally elevated LDL-C levels accompany FH as a result and when left untreated, leads to premature morbidity and mortality from ASCVD.15
Homozygous FH
Homozygous familial hypercholesterolemia (HoFH) is the more severe FH variant with abnormal genes inherited from both parents. It is extremely rare, with an incidence of approximately 1 in 1 million persons. Total cholesterol in individuals with HoFH usually ranges from 650 to 1000 mg/dL.15 A greater than or equal to 4-fold increase in plasma LDL-C levels are present at birth. These high LDL-C levels result in cholesterol deposits in cutaneous tissues, tendons, and vasculature, leading to severe and extensive atherosclerosis at a young age. If left untreated, patients with LDLR-negative HoFH often die before their second decade, and LDLR-defective patients develop significant atherosclerotic disease by age 30.13
In patients with HoFH, clinical hallmark characteristics usually appear between age 10 and 20 years. The presence of an untreated LDL-C greater than 500 mg/dL and the existence of cutaneous xanthomas before 10 years of age indicate HoFH. Interdigital xanthomas, particularly between the index finger and thumb, are characteristic of HoFH. Atherosclerotic severity corresponds with the duration and extent of LDL-C level elevation.13
Heterozygous FH
Heterozygous familial hypercholesterolemia (HeFH) is a common congenital metabolic disorder, occurring in approximately 1 in 200 to 500 individuals or sometimes higher in certain populations.13 It results from an inherited mutation from one parent, with total cholesterol concentrations in the range of 350 to 550 mg/dL.15 HeFH is usually a silent disease, with the only clinical presentation being the presence of a significantly elevated LDL-C (generally greater than 190 mg/dL in untreated adults and greater than 160 mg/dL in untreated children or adolescents). The presence of subclinical atherosclerosis may occur in patients with HeFH, with carotid intima-media thickening and aortic lesion detectable by magnetic resonance imaging at approximately 8 to 10 years of age. About 1 in 4 adolescents will have detectable coronary artery calcium (CAC).13
During adulthood, tendon xanthomas and corneal arcus may occur after sustained LDL-C elevations. Unfortunately, angina or premature MI are usually the primary CV signs of HeFH, sometimes occurring in the patient’s thirties. The clinical course of HeFH is variable, but higher LDL-C levels and additional risk factors correlate with a greater CV risk.13
Diagnosis of FH
The clinical diagnosis of FH depends on the presence of a combination of physical findings, personal or family history of hypercholesterolemia, premature ASCVD, and elevated LDL-C levels. Genetic testing may confirm FH, but the absence of a detection of a mutation does not exclude diagnosis. A substantial percentage of patients with a definitive clinical diagnosis of FH may have an unidentified mutation or a polygenic mutation.16 A recent study indicated genetic testing helps guide cardiac risk assessment. The presence of an FH mutation may confer significantly greater risk. Studies showed that individuals with an LDL-C level equal to or greater than 190 mg/dL with no FH mutation had a 6-fold increased risk of coronary artery disease (CAD), while those with an FH mutation had a 22-fold higher risk versus the reference group (those with an LDL-C ≤130 mg/dL).17
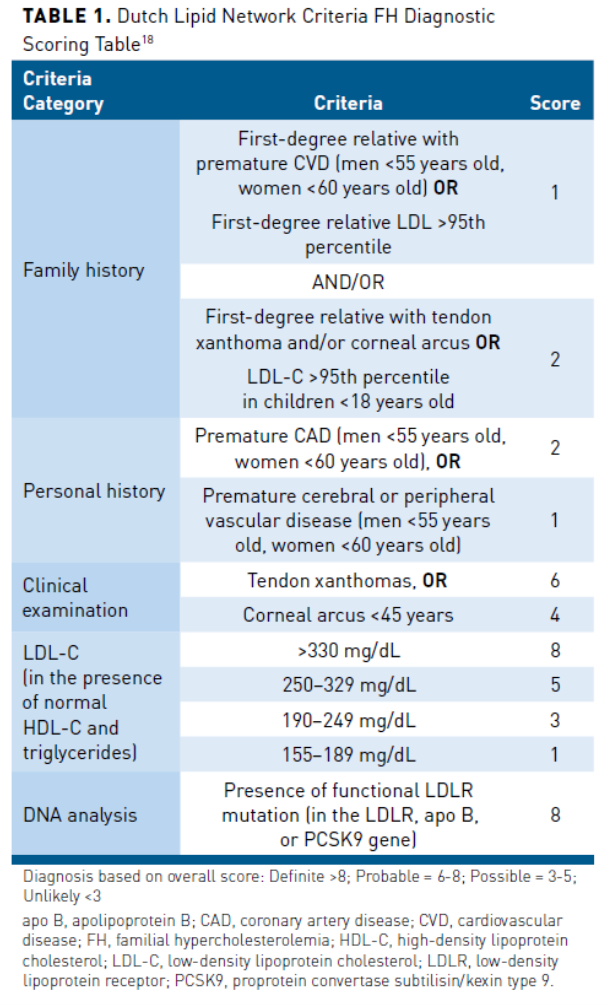
There is a lack of uniform FH diagnostic criteria. There are, however, several diagnostic tools with comparable predictive value for the diagnosis of FH. The primary diagnostic tools include the US Make Early Diagnosis to Prevent Early Death (MEDPED) criteria, the Dutch Lipid Clinic Network criteria, the UK Simon Broome system, and the National Lipid Association expert panel recommendations. The only tools incorporating genetic testing results into their diagnostic criteria are the Simon Broome and the Dutch Lipid Clinic Network criteria.16 The Dutch Lipid Clinic Network uses a scoring system to predict the likelihood of FH (Table 118), and diagnostic criteria require a positive genetic test and an additional indicator, such as elevated LDL-C, to confirm FH diagnosis.18 Positive genetic testing results correlate to a definitive FH diagnosis using the Simon Broome Criteria.16
FH Clinical and Financial Burden
An observational, retrospective study using electronic health system data reported the MACE (all-cause death, MI, percutaneous coronary intervention, or coronary artery bypass) and medical care costs associated with FH. An internally validated electronic health record-based algorithm using the modified Dutch Lipid Clinic Network criteria categorized patients as definite, probable, possible, and unlikely FH, with the first 3 groups together representing the FH cohort. Of the 1.18 million patients, 205,290 patients had hyperlipidemia (non-FH cohort), and 32,613 had FH. The FH cohort primarily comprised patients with possible FH (93%), followed by those with probable FH (6%), and finally patients with definite FH (1%). The FH cohort experienced a MACE at a younger age (54 years vs 67 years; P <.001). After adjusting for risk factors, FH was significantly correlated with increased risk for MACE (OR, 4.02; 95% CI, 3.88-4.16; P <.0001) and mortality (OR, 1.20; 95% CI, 1.15-1.26; P <.0001) than the non-FH group. The FH cohort also had a higher total adjusted revenue per year (incidence rate ratio, 1.30 (95% CI, 1.28-1.33; P <.0001) than the non-FH group. This study showed a statistically significant correlation with FH and increased risk of adverse CV outcomes and increased health care costs versus those with hyperlipidemia without FH.19
Practice Gaps
Although optimized statin therapy, including appropriate agent, dose, and medication adherence, is first line, some patients require adjunctive nonstatin treatment to reach LDL-C goals. This is especially true in high-risk patients. A study conducted in 2,585,931 US patients enrolled in the PINNACLE national registry during 2017-2018 examined LDL-C goal achievement in patients with established ASCVD.The study found large gaps between guideline recommendations and goal attainment.20 The 2018 American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Management of Blood Cholesterol is the most widely cited cholesterol guideline in the United States and recommends achieving LDL-C less than 70 mg/dL in patients with ASCVD.2 Approximately 53% of all patients with ASCVD in the PINNACLE registry study had no history of lipid-lowering medication. Among the 1,081,613 patients receiving statin monotherapy, 31.9% achieved an LDL-C less than 70 mg/dL, 41.7% an LDL-C of 70-100 mg/dL, and 26.4% an LDL-C equal to or greater than 100 mg/dL.20 A recent consensus statement by the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) on the management of hyperlipidemia recommendseven more aggressive LDL-C goals/thresholds than the 2018 ACC/AHA guideline, particularly in patients with the highest CV risk (Table 22,21,22).
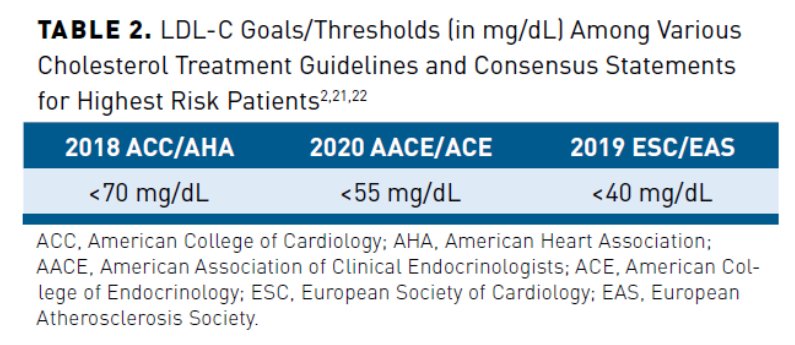
Europe has some of the most aggressive cholesterol treatment guidelines, with the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) recommending LDL-C of less than 55 mg/dL in very high-risk patients and less than 40 mg/dL in the highest risk patients (ie, recurrent vascular event within 2 years) (Table 22,21,22). The European Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care (DA VINCI) study included 3000 primary prevention patients and 2888 secondary prevention patients from 18 European countries on lipid-lowering therapy between June 2017 and November 2018.23 It measured the proportion of patients receiving stabilized lipid-lowering therapy (LLT) reaching their LDL-C risk-based goal recommended by 2016 ESC/EAS guidelines. Eighty-three percent received statin monotherapy, 9% of patients received a moderate- or high-intensity statin with ezetimibe, and 1% received a PCSK9 inhibitor with a statin and/or ezetimibe. As the updated ESC/EAS guidelines were published after the study’s completion, a post hoc analysis of the proportion of patients achieving the 2019 ESC/EAS goals was performed. Overall, 33% of patients achieved the 2019 ESC/EAS LDL-C guideline goals. For the very high-risk cohort, the LDL-C goal was attained by 22% of patients on high-intensity statin monotherapy, 20% of patients on ezetimibe combination therapy, and 58% of patients on PCSK9 inhibitor combination therapy. The results of DA VINCI highlight missed opportunities to optimize LDL-C in clinical practice and demonstrate the challenge of reaching these more aggressive LDL-C targets with statin monotherapy.
Two recent simulation model studies investigated the potential role of PCSK9 inhibitor therapy in patients with ASCVD to reach treatment goals in combination with other lipid-lowering treatment and in cases of statin intolerance. Researchers conducted a study of 105,269 patients using an administrative database of US medical and pharmacy claims from January 1, 2012, through December 21, 2013, to estimate the percentage of patients with ASCVD requiring a PCSK9 inhibitor after maximal intensification of LLT. The stepwise treatment intensification logic applied by simulation was as follows: statin-naïve patients received atorvastatin 20 mg, up-titration to atorvastatin 80 mg, ezetimibe added, alirocumab 75 mg added, and up-titration to alirocumab 150 mg. At baseline, approximately 51% of patients in the database cohort used statins alone, and 2% used statins plus ezetimibe, with 35% achieving an LDL-C less than 70 mg/dL in the overall cohort. After simulated treatment intensification, approximately 99% could achieve an LDL-C target of less than 70 mg/dL, with 67.3% receiving statin monotherapy, 18.7% receiving statins plus ezetimibe, and 14% receiving add-on alirocumab. A follow-up simulation study sought to estimate the utilization of LLT needed to reach treatment goals after accounting for statin intolerance. Allowing for a 10% incidence of complete statin intolerance, the need for PCSK9 inhibitor use increased by 5.7% in this simulation. In summary, these studies predict that approximately 20% of patients with ASCVD will require a PCSK9 inhibitor to achieve LDL-C less than 70 mg/dL despite statin optimization and the use of ezetimibe.24,25
Discovery of PCSK9
The search for genetic mutations causing FH led to identifying gain-of-function mutations in PCSK9 as a cause of FH. Further research discovered inactivating mutations resulting in increased LDLR function and lower LDL-C levels, leading to the development of PCSK9 inhibitors as drugs to treat hyperlipidemia.26 PCSK9 levels are the most robust regulator of cholesterol in the body. PCSK9 inhibitors, alirocumab and evolocumab, are human monoclonal antibodies that bind in a 1:1 ratio with circulating PCSK9, preventing binding to the LDLR. This binding creates decreased levels of PCSK9, resulting in an accumulation of LDLR and accelerated clearance of LDL-C.14 The addition of a PCSK9 inhibitor to a statin can further reduce LDL-C levels by 43% to 64%. The development of PCSK9 inhibitors represented a significant advancement in the treatment of hyperlipidemia, providing high-risk patients effective LDL-C−lowering therapy when statin monotherapy or combination therapy with ezetimibe is suboptimal or not tolerated.2 Evolocumab and alirocumab are available as subcutaneous injection formulations and FDA approved to treat primary hyperlipidemia, including FH, as an adjunct to diet, alone, or combined with other LLT to reduce LDL-C.27,28 They are also approved to reduce the risk of MACE in adults with established ASCVD. The PCSK9 inhibitors have a favorable adverse effect profile. The ODYSSEY OUTCOMES and FOURIER trials found no difference between the PCSK9 inhibitors and placebo in terms of myalgia, liver function test elevations, and new-onset diabetes, adverse effects that have been associated with statin use. Nasopharyngitis and mild injection-site reactions are some of the most commonly reported adverse effects. Even at very low LDL-C levels achieved in studies, no increase in neurocognitive treatment-emergent adverse reactions was observed.29
Barriers to Treatment With PCSK9 Inhibitors
Despite their demonstrated safety profile and efficacy in reducing LDL-C and MACE, PCSK9 inhibitors are used sparingly in real-world clinical practice. Recent retrospective studies reveal that only about 1% of patients with ASCVD or FH are prescribed a PCSK9 inhibitor.23,30 Some possible barriers to clinical acceptance of PCSK9 inhibitor treatment include lack of patient and provider acceptance, high out-of-pocket cost, and pharmacy benefit access issues.
Therapeutic inertia may explain, in part, failure to start or add on new therapy when there is suboptimal control with current medication treatment. Both patient and provider factors can contribute to reluctance to begin new medications. Changing medications may be confusing for patients who have been taking the same drugs for years. Patients may be fearful of adverse effects or using injectable medications and may lack an understanding of risk versus benefits. Providers may share some of these fears and hesitate to intensify therapy.31
Other factors, including the cost of therapy and pharmacy benefit coverage, play a role in the low utilization rates of PCSK9 inhibitors. The wholesale acquisition cost of alirocumab and evolocumab, when first approved, exceeded $14,000 per year.31 As a result of these agents’ high price and to ensure use in appropriate patients, many payers limited utilization of PCSK9 inhibitors through step therapy, prior authorization (PA) requirements, and cost-sharing procedures resulting in high co-pays.31,32
An analysis of 2016 formulary coverage and PA data from an extensive US database revealed burdensome PA requirements for PCSK9 inhibitors. Between 82% and 97% required a PA for the coverage of PCSK9 inhibitors, restricting prescribing to a specialist in 32% to 66% of plans. Payers required submission of medical record documentation in 40% to 75% of cases and frequently mandated genetic testing or meeting clinical diagnostic tool criteria for FH. Consistent with guideline recommendations, concomitant statin therapy was required unless contraindicated or patient was intolerant. Payers also frequently required recent LDL-C levels.33
An observational analysis of 45,029 patients in the United States used a pharmacy claims database and electronic medical record to examine the association between PA and co-pay with patient access to newly prescribed PCSK9 inhibitors between August 1, 2015, and July 31, 2016. Of the percentage of patients approved on day 1 (20.8%) and rejected on day 1 (79.2%), 73.5% of claims were resubmitted or appealed, which resulted in a final approval rate of 47.2%. The median time between initial PA submission and approval was 3.9 days but varied dramatically, with 17.2% waiting 30 days or more. Of the 47.2% of approvals, 34.7% of patients never picked up the medication. The median co-pay was $15 (range, $0-$2822) per month supply in the abandoned prescription group. After adjusting for patient demographics, prescriber, payer, and pharmacy, the co-pay amount significantly predicted whether the patient would pick up medication after approval ($10 co-pay OR 0.81; 95% CI, 0.81-0.82; $100 co-pay OR 0.16; 95% CI, 0.15-0.17; vs no co-pay).34
A study using a sensitivity analysis examined the impact of rejected or never-received PCSK9 inhibitor claims on CV outcomes in 139,036 individuals between August 2015 and December 2017. A significantly increased risk for composite CVE outcome was correlated with never-received and rejected claims versus paid PCSK9 inhibitor claims (paid claim defined as 338 or more days of PCSK9 inhibitor treatment within 12 months) in a propensity score-matched analysis (never received HR, 1.21; 95% CI, 1.04-1.38; P = .03; rejected claims HR, 1.16; 95% CI, 1.02-1.30; P = .04).35
Of note, these studies evaluated prescription patterns before CV outcomes data for PCSK9 inhibitors were published, which may have affected the prescribing rate and payer approval criteria.10,11,34 Additionally, the manufacturers of alirocumab and evolocumab cut drug prices by 60% in October 2018 and March 2019 to approximately $5850 per year.36,37 This price reduction may also result in increased utilization due to the lowering of patients’ out-of-pocket cost, particularly for patients who pay coinsurance as a percentage of the drug’s cost. After accounting for these changes, it is likely that significant barriers to access still exist.
Identifying these barriers to PCSK9 inhibitor therapy access is instrumental in developing strategies to overcome them to reduce patient clinical and financial burden. Managed care pharmacists can help educate healthcare provider staff regarding specific PA criteria (eg, diagnostic criteria, trial of statins, trial of ezetimibe, labs required) and necessary documentation to streamline the PA process. Education of patients, medication therapy management, and implementation of clinical programs can also improve patient adherence.38-43 Pharmacists are also well-positioned to assist patients who have difficulty affording PCSK9 inhibitors by referring them to financial assistance such as the manufacturers’ patient assistance programs or co-pay cards.
Conclusions
Hyperlipidemia and ASCVD are prevalent diseases associated with significant morbidity, mortality, and financial burdens. Reduction of LDL-C results in decreased rates of CV events, with guidelines targeting lower levels for patients at the highest CV risk. Statin therapy is the first-line treatment of hyperlipidemia. However, a significant portion of patients are unable to attain optimal LDL-C control on statin monotherapy or combination therapy with ezetimibe and would benefit from a PCSK9 inhibitor. Despite the PCSK9 inhibitors’ ability to effectively lower LDL-C and reduce the risk of adverse CV effects, significant barriers to their use exist. Managed care pharmacists can help address these barriers to improve patient care and outcomes.
Author affiliation: Paul B. Shaw, PharmD, BCPS, BCCP, is clinical pharmacy specialist−cardiology, Kaiser Permanente of Colorado, Clinical Assistant Professor – University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Lafayette, CO.
Funding source: This activity is supported by educational funding provided by Amgen.
Author disclosure: Dr Shaw has no relevant financial relationships with commercial interests to disclose.
Authorship information: Analysis and interpretation of data; concept and design; critical revision of the manuscript for important intellectual content; drafting of the manuscript; supervision.
Address correspondence to: paul.b.shaw@kp.org
Medical writing and editorial support provided by: Lori Uildriks, PharmD, BCPS, BCGP
References
1. Heart disease and stroke. Centers for Disease Control and Prevention (CDC). Reviewed October 7, 2020. Accessed December 28, 2020. cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm
2. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [Erratum in: Circulation. 2019;139(25):e1182-e1186]. Circulation. 2019;139(25):e1082-e1143. doi: 10.1161/CIR.0000000000000625
3. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [Erratum in: J Am Coll Cardiol. 2019;74(10):1428-1429. Erratum in: J Am Coll Cardiol. 2020;75(7):840]. J Am Coll Cardiol. 2019;74(10):1376-1414. doi: 10.1016/j.jacc.2019.03.009
4. Virani SS, Alonso A, Benjamin EJ, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. doi: 10.1161/CIR.0000000000000757
5. Fox KM, Wang L, Gandra SR, Quek RG, Li L, Baser O. Clinical and economic burden associated with cardiovascular events among patients with hyperlipidemia: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16:13. doi: 10.1186/s12872-016-0190-x
6. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. doi: 10.1093/eurheartj/ehx144
7. Jarcho JA, Keaney JF Jr. Proof that lower is better—LDL cholesterol and IMPROVE-IT. N Engl J Med. 2015;372(25):2448-2450. doi: 10.1056/NEJMe1507041
8. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5
9. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489
10. Schwartz GG, Steg PG, Szarek M, et al. ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174
11. Sabatine MS, Giugliano RP, Keech AC, et al. FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664
12. Wilemon KA, Patel J, Aguilar-Salinas C, et al. Representatives of the Global Familial Hypercholesterolemia Community. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol. 2020;5(2):217-229. doi: 10.1001/jamacardio.2019.5173
13. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The Agenda for Familial Hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132(22):2167-2192. doi: 10.1161/CIR.0000000000000297
14. Rosenson RS, Hegele RA, Fazio S, Cannon CP. The evolving future of PCSK9 inhibitors. J Am Coll Cardiol. 2018;72(3):314-329. doi: 10.1016/j.jacc.2018.04.054
15. Goldberg AC, Hopkins PN, Toth PP, et al; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association expert panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5(3 suppl): S1-S8. doi: 10.1016/j.jacl.2011.04.003
16. McGowan MP, Dehkordi SH, Moriarty PM, Duell PB. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2019;8(24):e013225. doi: 10.1161/JAHA.119.013225
17. Khera AV, Won HH, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67(22):2578-2589. doi: 10.1016/j.jacc.2016.03.520
18. World Health Organization. Familial hypercholesterolaemia (FH): report of a second WHO consultation. September 4, 1998. Accessed December 30, 2020. whqlibdoc.who.int/hq/1999/WHO_HGN_FH_CONS_99.2.pdf
19. Patel P, Hu Y, Kolinovsky A, et al. Hidden burden of electronic health record-identified familial hypercholesterolemia: clinical outcomes and cost of medical care. J Am Heart Assoc. 2019;8(13):e011822. doi: 10.1161/JAHA.118.011822
20. Allen M, Arnold SV, Lohr NL, et al. Abstract 12904: Assessing low-density lipoprotein cholesterol risk in secondary prevention patients within the PINNACLE National Outpatient Registry. Circulation. 2019;140:A12904.
21. Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and the prevention of cardiovascular disease algorithm. Endo Pract. 2020;26:1-29.
22. Mach F, Baigent C, Catapano AL, et al. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455
23. Ray KK, Molemans B, Schoonen WM, et al; DA VINCI study. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DA VINCI study. Eur JPrev Cardiol. Published online August 28, 2020. doi: 10.1093/eurjpc/zwaa047
24. Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(9):959-966. doi: 10.1001/jamacardio.2017.2289
25. Cannon CP, Sanchez RJ, Klimchak AC, et al. Simulation of the impact of statin intolerance on the need for ezetimibe and/or proprotein convertase subtilisin/kexin type 9 inhibitor for meeting low-density lipoprotein cholesterol goals in a population with atherosclerotic cardiovascular disease. Am J Cardiol. 2019;123(8):1202-1207. doi: 10.1016/j.amjcard.2019.01.028
26. Kim EJ, Wierzbicki AS. The history of proprotein convertase subtilisin kexin-9 inhibitors and their role in the treatment of cardiovascular disease. Ther Adv Chronic Dis. 2020;11: 2040622320924569. doi: 10.1177/2040622320924569
27. Praluent. Prescribing information. Regneron Pharmaceuticals, Inc; 2020. Accessed February 23, 2021.regeneron.com/sites/default/files/Praluent_PI.pdf
28. Repatha. Prescribing information. Amgen Inc; 2020. Accessed February 23, 2021. pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/repatha_pi_hcp_english1-5.ashx
29. Giugliano RP, Mach F, Zavitz K, et al; EBBINGHAUS Investigators. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633-643. doi: 10.1056/NEJMoa1701131
30. Chamberlain AM, Gong Y, Shaw KM, et al. PCSK9 inhibitor use in the real world: data from the national patient-centered research network. J Am Heart Assoc. 2019;8(9):e011246. doi: 10.1161/JAHA.118.011246
31. Bergethon KE, Wasfy JH. Increasing the adoption and diffusion of a novel pharmacological therapy that is both mortality reducing and cost-effective. J Am Heart Assoc. 2019;8(3):e011783. doi: 10.1161/JAHA.118.011783
32. Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243-254. doi: 10.1002/clc.22713
33. Doshi JA, Puckett JT, Parmacek MS, Rader DJ. Prior authorization requirements for proprotein convertase subtilisin/kexin type 9 inhibitors across US private and public payers. Circ Cardiovasc Qual Outcomes. 2018;11(1):e003939. doi: 10.1161/CIRCOUTCOMES.117.003939
34. Navar AM, Taylor B, Mulder H, et al. Association of prior authorization and out-of-pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiol. 2017;2(11):1217-1225. doi: 10.1001/jamacardio.2017.3451
35. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitor on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi: 10.1161/CIRCOUTCOMES.118.005404
36. Amgen makes Repatha (evolocumab) available in the US at a 60 percent reduced list price. News release. Amgen; October 24, 2018. Accessed December 28, 2020. amgen.com/media/news-releases/2018/10/amgen-makes-repatha-evolocumab-available-in-the-us-at-a-60-percent-reduced-list-price/
37. Regeneron and Sanofi offer Praluent (alirocumab) at a new reduced US list price. News release. Regeneron; February 11, 2019. Accessed December 28, 2020. investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-offer-praluentr-alirocumab-new-reduced-us
38. Taitel M, Jiang J, Rudkin K, Ewing S, Duncan I. The impact of pharmacist face-to-face counseling to improve medication adherence among patients initiating statin therapy. Patient Prefer Adherence. 2012;6:323-329. doi: 10.2147/PPA.S29353
39. Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340-349. doi: 10.1001/jamainternmed.2015.7667
40. Mann A, Esse TW, Serna O, Castel LD, Abughosh SM. Effectiveness of mailed letters to improve medication adherence among Medicare Advantage Plan participants with chronic conditions. Patient Prefer Adherence. 2018;13:37-46. doi: 10.2147/PPA.S185848
41. Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med. 2013;173(1):38-43. doi: 10.1001/2013.jamainternmed.717
42. Spears J, Erkens J, Misquitta C, Cutler T, Stebbins M. A pharmacist-led, patient-centered program incorporating motivational interviewing for behavior change to improve adherence rates and star ratings in a Medicare plan. J Manag Care Spec Pharm. 2020;26(1):35-41. doi: 10.18553/jmcp.2020.26.1.35
43. Ferries E, Dye JT, Hall B, Ndehi L, Schwab P, Vaccaro J. Comparison of medication therapy management services and their effects on health care utilization and medication adherence. J Manag Care Spec Pharm. 2019;25(6):688-695. doi: 10.18553/jmcp.2019.25.6.688
