- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Heart Failure With Reduced Ejection Fraction: Clinical and Economic Burden and Insights into Current and Emerging Treatments
ABSTRACT
Heart failure (HF) imposes a large and growing burden on the population, with a prevalence that is projected to increase to more than 8 million adults by 2030. The high risk of morbidity and mortality associated with HF is further exacerbated by the frequent presence of comorbidities. The coexistence of HF and comorbid conditions can result in emergency department visits and hospitalizations that not only affect patients and their families but also pose a growing economic burden on health care systems. The largest costs arise from hospitalization for HF, with outpatient care and associated medication costs comprising the second largest component. For patients with HF with reduced ejection fraction (HFrEF), defined as left ventricular ejection fraction of 40% or less, remarkable improvements in outcomes have been observed in recent decades due to the availability of disease-modifying therapies. However, the management of HFrEF remains suboptimal, with many patients either not receiving guideline-directed medical therapy (GDMT) or experiencing delays in receiving target doses. Since this can result in preventable hospitalizations and deaths, action is needed to ensure rapid initiation of GDMT. Optimal treatment can be hindered by such patient factors as the presence of comorbidities and socioeconomic barriers that include the cost of multiple treatments. Furthermore, poor treatment adherence is common among patients with HF. Measures aimed at tailoring therapies to individual patients and reducing medical costs are important to increase the uptake of and adherence to therapy and therefore improve clinical outcomes.
Am J Manag Care. 2023;29:S180-S186. https://doi.org/10.37765/ajmc.2023.89415
For author information and disclosures, see end of text.

Introduction
Heart failure (HF) is defined as a clinical syndrome, although a lack of consensus around a clear definition has led to challenges in characterizing the disease within clinical practice and clinical research. At present, there remains substantial heterogeneity in the diagnostic criteria for HF, which may include metabolic, hemodynamic, and symptom-based parameters. Most HF trials include patients who are identified using a threshold of left ventricular ejection fraction (LVEF) or specific New York Heart Association (NYHA) functional class categories (classes I-IV indicating symptom severity).1 However, the Heart Failure Society of America (HFSA) recently proposed a universal definition and classification of HF that is based on symptoms and/or signs resulting from a structural and/or functional cardiac abnormality and that is supported by elevated natriuretic peptide levels and/or evidence of pulmonary or systemic congestion (Figure 1).2
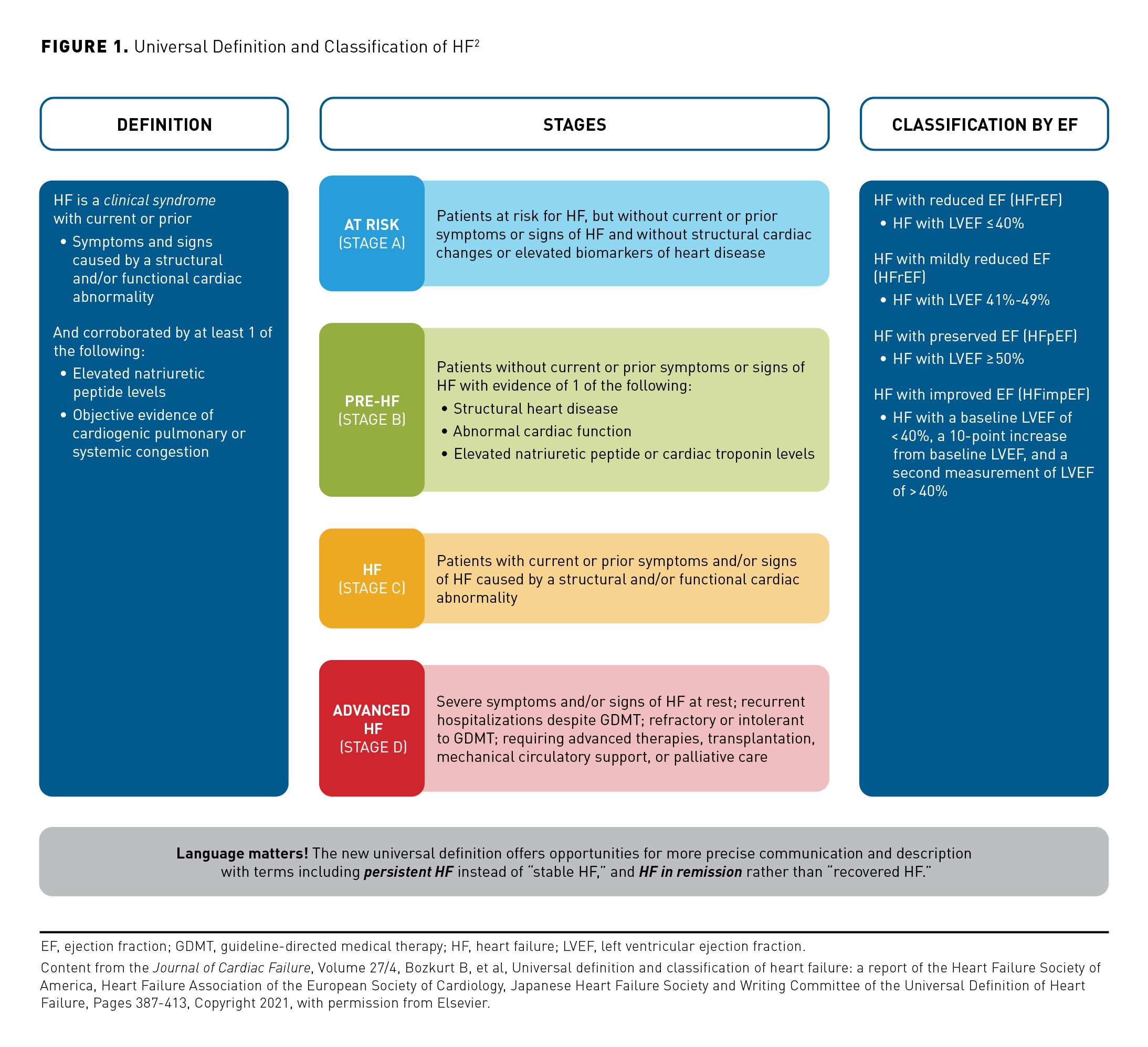
The latest joint guideline from the American College of Cardiology (ACC), American Heart Association (AHA), and HFSA for the management of HF gives treatment recommendations according to this modified version of the classic definition of HF.3 The new classification defines HF with reduced ejection fraction (HFrEF) as LVEF 40% or less, and patients with HF and an EF above 40% and below 50% are classified as having HF with mildly reduced EF.3 Patients with a previous diagnosis of HFrEF but with a follow-up LVEF of more than 40% are now described as having HF with improved EF. This change reflects the dynamic nature of HFrEF, where improvement or deterioration can occur over time.
HF imposes one of the highest disease burdens of any condition in the United States, with around 6.5 million Americans 20 years or older reported to be affected.4 This figure was calculated using data from 2011 to 2014, and it continues to grow, largely as a result of aging of the population. From 2012 to 2030, the prevalence of HF is projected to increase by 46% to more than 8 million people at least 18 years of age.4
Although the survival of patients with HF has improved in recent decades, 1-year mortality remains high at 29.6%.5 Similarly, data from the Atherosclerosis Risk in Communities study showed 30-day, 1-year, and 5-year case fatality rates after hospitalization to be 10.4%, 22%, and 42.3%, respectively.6 The clinical burden of HF is further increased by the frequent presence of comorbid conditions, including hypertension, hyperlipidemia, cardiac arrhythmias, coronary artery disease, type 2 diabetes (T2D), chronic obstructive airway disease, mental depression, and chronic kidney disease.7 Over 70% of patients with HF have comorbid conditions that independently increase the risk of morbidity, reduce quality of life, and increase mortality.8 Furthermore, the coexistence of HF and comorbid conditions can result in emergency department visits and hospitalizations that not only have an impact on patients and their families but also on the health care system as a whole.
Economic Burden
HF imposes a large and growing economic burden on health care systems, mostly as a result of aging of the population.9 Associated total medical costs from 2012 to 2030 are projected to increase from $21 billion to $53 billion.10 The estimated annual health care costs for HF—which include direct and indirect costs—are expected to reach $70 billion by 2030.10 The majority of these costs result from hospitalization for HF, which occurs frequently among affected patients.11,12 For each person with HF, the annual costs of care are estimated to be almost $30,000, half of which is accounted for by the cost of inpatient care, and a further quarter due to outpatient care.9 Approximately 30% to 40% of individuals with HF have a history of hospitalization.10,11,13 Furthermore, a study of national trends for inpatient HF care reported that 1.2 million hospitalizations occurred among 924,000 patients in 2017.14 Following hospital admission, patients with HF are at increased risk of all-cause readmission, which is estimated to be around 20% within 30 days of initial hospital admission.15,16 Outpatient care, including medication costs, is the second largest component of health care expenditures in HF.12 Other direct costs include rehabilitation, nursing care, and informal care.12 Given the health care costs associated with HF, measures aimed at reducing symptoms and improving long-term outcomes for patients with HF may have important benefits for both the health care system and for individual patients.
Current Treatments
Over the past 2 decades, there have been remarkable improvements in outcomes for patients with HFrEF, largely due to changes in clinical practice based on the results of several landmark trials of treatments involving renin-angiotensin system blockade17-23 and β-blockade.24-26 The use of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or angiotensin-neprilysin inhibitors (ARNIs) is now recommended as first-line therapy for patients with HF and HFrEF (Figure 2).3 This recommendation is based on results from large-scale clinical trials of both ACEIs and ARBs in the management of HFrEF, which demonstrated significant reductions in mortality and morbidity versus standard care.17-23
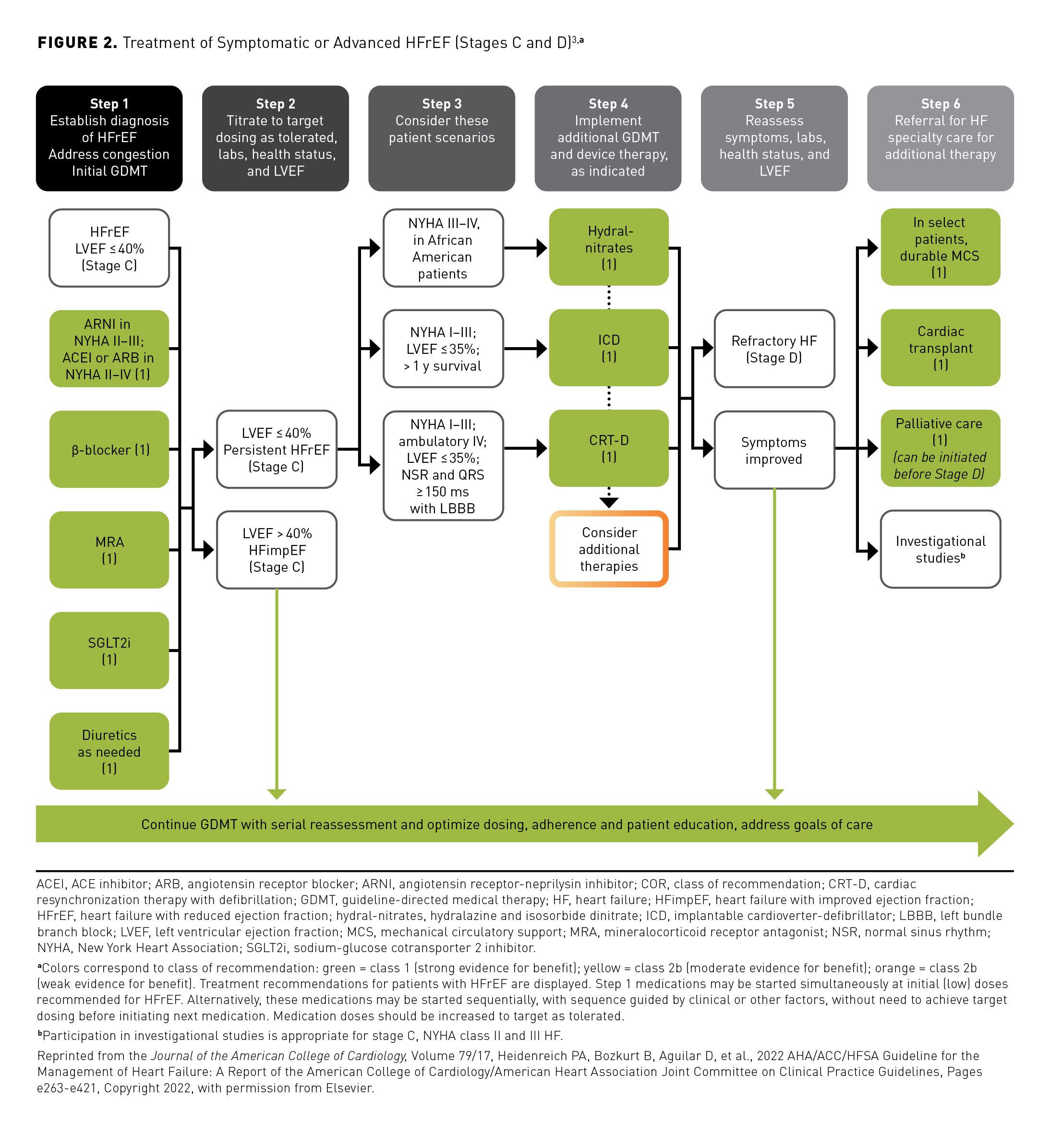
For patients with chronic symptomatic HFrEF with NYHA class II or III symptoms who can tolerate ACEI or ARB therapy, current guidelines advise switching to treatment with an ARNI due to evidence of improved morbidity and mortality with their use.3,27 Additionally, an ARNI is recommended for de novo treatment of HFrEF based on evidence that use of these agents enhances health status and improves prognostic markers and LV remodeling parameters when compared with ACEI or ARB therapy.3,28 β-blockers are also recommended for patients with current or previous symptoms of HFrEF; specifically, use of bisoprolol, carvedilol, or sustained-release metoprolol succinate improves LVEF and clinical status and reduces HF symptoms, hospitalizations, and mortality.3,24-26 These agents are also associated with improvements in LVEF, reduction in HF symptoms, and improved clinical status.29-31 Use of mineralocorticoid receptor antagonists (MRAs), members of the third group of agents recommended for patients with HFrEF, is associated with a reduced risk of mortality and hospitalizations.32-34 More recently, a fourth group of agents, the sodium-glucose cotransporter 2 inhibitors (SGLT2is), has produced benefits in patients with HF irrespective of the presence of T2D.35,36 The outcomes of 2 landmark trials (DAPA-HF [NCT03036124] and EMPEROR-Reduced [NCT03057977]) showed that inhibition of SGLT2 was associated with an approximate 25% reduction in a composite of cardiovascular death or HF hospitalization and an approximate 30% reduction in HF hospitalization when compared with placebo.35-37 Furthermore, the risk of a decline in renal function (as indicated by a composite of chronic dialysis, renal transplantation, or a ≥ 50% sustained reduction of estimated glomerular filtration rate) was significantly reduced by SGLT2 inhibition.37 Results of the DELIVER trial (NCT03619213) have shown that inhibition of SGLT2 can improve outcomes for patients with HF and mildly reduced or preserved EF (LVEF > 40%) with fewer worsening HF events and cardiovascular deaths and a reduced symptom burden when compared with placebo.38
Based on current evidence, clinical guidelines strongly recommend the use of these 4 groups of disease-modifying agents—β-blockers, ARNI, MRAs, and SGLT2is—as first-line treatments for HFrEF.3 Patients should receive multiple medications, as tolerated, to improve clinical outcomes. To ensure that patients are discharged on optimal therapy, guideline-directed medical therapy (GDMT) for HFrEF should be initiated during hospitalization following clinical stabilization.3
For patients with HFrEF (class III-IV) who self-identify as being Black, the addition of a combination of hydralazine and isosorbide dinitrate to optimal medical therapy is recommended.3 This guidance is based on the findings of 2 pivotal clinical trials showing that this combination was associated with reduced symptoms, morbidity, and mortality in Black patients.39-41 However, the benefits observed in these trials were higher than those typically achieved in clinical practice, and this regimen has not been widely used due to difficulties with treatment adherence, possibly related to treatment adverse effects.3,42
Heart rate reduction with the selective sinus node If current inhibitor, ivabradine, is also recommended for the management of patients with HFrEF (NYHA class II-III).3 The latest ACC/AHA/HFSA guideline recommends the addition of ivabradine to GDMT that includes a β-blocker at the maximum tolerated dose for patients with HFrEF (LVEF ≤ 35%) who are in sinus rhythm with evidence of an elevated resting heart rate (≥ 70 beats/min).3 For these individuals, ivabradine use reduces the risk of HF hospitalizations and cardiovascular death.43
The role of standard GDMT is less well-established for patients with advanced HFrEF due to limited clinical experience in this population. The use of sacubitril/valsartan is not currently endorsed by practice guidelines for use in patients with class IV HFrEF, and the results of a recent clinical trial of sacubitril/valsartan versus valsartan in patients with advanced HFrEF (class IV), HFN-LIFE (NCT02816736), showed that adding sacubitril as assessed by NT-proBNP levels provides no benefit.44 Therapeutic options for advanced HF include durable mechanical support with LV assist devices (LVADs) and transplant in addition to palliative care.3 Survival for patients with advanced HF (class IV) has substantially improved in recent years with the introduction of new-generation LVADs.45
Optimizing Existing Therapies
To date, clinical management of HFrEF has generally involved initiating treatments in a sequence that largely follows the chronological order of published pivotal trials (ie, starting with ACEIs or ARBs, then adding a β-blocker, then an MRA, switching to an ARNI, and finally adding an SGLT2i).46 This approach also involved a cautious and gradual increase in dosage until the recommended level was reached before adding another therapy. More recently, it was suggested that patient outcomes could be improved and hospitalizations could be reduced by using alternative approaches to treatment sequencing.47-51 However, the optimal approach to sequencing of therapy has not been established.
The ACC/AHA/HFSA guideline states that GDMT is the foundation of care for patients with HFrEF and should be started as soon as possible after diagnosis.3,52 However, accelerated initiation of therapy could be beneficial; the effects of individual agents are independent and additive, with each drug conferring benefit soon after initiation (within 30 days).3,46,51 A modeling study based on data from pivotal trials showed that simply shortening the time to gradually increase the dose of each drug reduced the occurrence of hospitalizations and deaths.46 This finding is to be expected given the known early benefits associated with the 4 first-line agents. However, changing the sequencing of administration to start with either an SGLT2i or an MRA allowed rapid attainment of target doses and was associated with the greatest benefits in terms of hospitalization and mortality. In contrast, starting 2 drugs simultaneously produced only modest additional benefit.
Despite the existence of clinical guidelines, current evidence suggests that the management of HFrEF remains suboptimal.13 An analysis of data from the CHAMP-HF registry, which included US outpatients with HFrEF who were receiving at least 1 oral treatment for HF, identified substantial gaps in the use of GDMT.52 Among patients eligible for treatment, a substantial proportion was not prescribed ACEI/ARB/ARNI treatment, a β-blocker, or MRA therapy (27%, 33%, and 67%, respectively). When medications were prescribed, only a minority of patients received target doses (an ACEI/ARB, 17%; an ARNI, 14%; a β-blocker, 28%), although most received target doses of an MRA (77%). Furthermore, for patients eligible for all classes of medication, only 1% simultaneously received target doses of an ACEI/ARB/ARNI, a β-blocker, and an MRA, and less than 25% received any dose of all 3 medications. An analysis of CHAMP-HF registry data also showed that even if patients with HFrEF did receive GDMT, they frequently experienced delays in receiving target doses of treatment.53
Failure to start GDMT in the hospital means that the majority of patients (> 75%) will not receive appropriate therapy within the next year as outpatients.54 Since delaying treatment by even a few weeks can result in preventable hospitalizations and deaths, the use of simultaneous or rapid-sequence initiation of quadruple therapy should be considered a high priority. In the PROMPT-HF trial (NCT04514458), investigators evaluated the use of targeted and tailored electronic health record–based alerts aimed at improving GDMT use in eligible outpatients with HFrEF.55 The results showed that use of the alerting system was associated with significantly higher uptake of GDMT at 30 days versus usual care; it represents a low-cost intervention that could be rapidly integrated into clinical care.
Importantly, the likelihood of receiving target doses of GDMT can be reduced by several patient factors, including the presence of comorbidities particularly, renal disease and socioeconomic barriers (eg, the cost of multiple treatments, the ability of patients to attend frequent office visits for treatment optimization).52 Out-of-pocket expenses can be a barrier to quadruple therapy for some patients.56 This burden is likely to increase following the addition of information concerning newer therapies, such as ARNIs and SGLT2is, to clinical guidelines. In a recent analysis of data from the Medical Expenditure Panel Survey, one-third of patients with HF experienced subjective financial hardship from medical bills, with 13.2% being unable to pay medical bills at all.57 The risk of financial hardship was greatest for patients 65 years or younger (possibly related to their ineligibility for Medicare coverage) and for individuals with less education.56,57 High financial burden can have an adverse impact on health outcomes, because affected patients are more likely to miss clinic visits, report poorer quality of life, have a sedentary lifestyle, and experience housing instability.57
These findings indicate an urgent need for increased efforts to reduce out-of-pocket costs for patients with HF and evaluation of cost as a patient-reported outcome in future clinical trials. Involvement of specialist cardiac pharmacists can help in addressing the cost implications of treatment for patients, improving adherence with GDMT, and optimizing clinical outcomes, which, in turn, reduces the risk of HF exacerbations and hospital admissions.56 According to results of the survey discussed above, the high prevalence of financial hardship among patients despite insurance coverage (ie, only 2.4% of patients with HF were uninsured vs 9% of patients without HF) emphasizes the need to improve the quality of insurance coverage, particularly as newer drugs for HF are becoming available.57
The ACC/AHA/HFSA guideline recommends an individualized approach to the initiation and titration of GDMT for HFrEF. Treatment optimization should be undertaken as soon as possible, taking account of symptoms, vital signs, functional status, tolerance, renal function, electrolyte levels, comorbidities, underlying cause of HF, and availability of follow-up.3 However, further research is needed to determine how to individualize treatment for specific causes of HFrEF and to assess the role of genetic and other individual factors in patient management.
Overall, the highest risk for decompensation requiring readmission is seen in the days and weeks following hospital discharge.16 Therefore, referral to multidisciplinary HF disease management programs is recommended for patients with recurrent hospitalizations for HFrEF to reduce the risk of further hospitalization.3 In addition, follow-up in the early postdischarge period can help to optimize understanding of changes to the care plan resulting from hospital admission, and this has been associated with a reduced risk of subsequent rehospitalization.3
Future Treatments
Since poor adherence to treatment is common among patients with HF, it is important that clinicians have a range of therapeutic options to consider for individual patients to reduce the risk of symptom exacerbations, disease progression, and hospitalizations.58 The search for a treatment that can increase myocardial performance has continued for many decades, although no drug that directly improves systolic function has been shown to improve clinical outcomes, including survival.59 However, 1 such agent, the selective cardiac myosin activator omecamtiv mecarbil, has been shown to improve cardiac function in patients with HFrEF, although this drug is not currently approved by the FDA for clinical use. In a clinical study, GALACTIC-HF (NCT02929329), patients with an LVEF of 35% or less who were receiving GDMT and device therapy who were given omecamtiv mecarbil had a lower incidence of a composite of a HF event or death from cardiovascular causes when compared with a placebo group.60
The effect of vericiguat, a novel oral soluble guanylate cyclase stimulator, has also been recently evaluated in patients with HFrEF. Vericiguat was compared with placebo in relatively high-risk patients with HFrEF (LVEF < 45%) who were recently hospitalized or received intravenous diuretic therapy and were receiving GDMT.61 In this population of patients with HFrEF and poor prognosis, treatment with vericiguat was associated with reductions both in hospitalization for HF and in cardiovascular death. These encouraging findings will need further evaluation to determine the future role of this agent in the management of HFrEF. Research is also needed to develop methods to assess treatment adherence in clinical practice and to evaluate interventions that can improve clinical outcomes and readmission rates.58
Conclusions
In view of the current suboptimal treatment of patients with HFrEF, our approach toward medical management that combines access, affordability, and initiatives to initiate and titrate therapies with proven benefits at a patient, health system, and societal level should be reconsidered.13 Further research is needed to determine how to implement therapies that have proven benefits in HFrEF so that patients and the wider society can benefit from recent advances in medical care. An improved understanding of patient-level costs associated with HF therapies and measures that reduce these costs are important to increase the uptake of and adherence to therapy and to optimize clinical outcomes.
Acknowledgments
The author meets criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The author received no payment related to the development of the manuscript. Jennifer Garrett, MBBS, of Envision Pharma Group provided medical writing and editorial support, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and Lilly USA, LLC. BIPI and Lilly were given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author Affiliations: Yale University School of Medicine, New Haven, CT.
Funding Source: Publication of this article was supported by Boehringer Ingelheim Pharmaceuticals, Inc. and Lilly USA, LLC.
Author Disclosures: Dr Sen reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Analysis and interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content.
Address Correspondence to: Sounok Sen, MD. Yale University School of Medicine, 789 Howard Ave, New Haven, CT 06519. Email: sounok.sen@yale.edu
REFERENCES
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852. doi:10.1161/CIR.0b013e31829e8807
- Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):387-413. doi:10.1016/j.cardfail.2021.01.022
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421. doi:10.1016/j.jacc.2021.12.012
- Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
- Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016-1022. doi:10.1016/j.amjcard.2007.11.061
- Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med. 2015;128(1):38-45. doi:10.1016/j.amjmed.2014.08.024
- Ambrosy AP, Stevens SR, Al-Khalidi HR, et al; STICH Trial Investigators. Burden of medical co-morbidities and benefit from surgical revascularization in patients with ischaemic cardiomyopathy. Eur J Heart Fail. 2019;21(3):373-381. doi:10.1002/ejhf.1404
- Heidenreich PA, Fonarow GC, Opsha Y, et al; HFSA Scientific Statement Committee Members Chair. Economic issues in heart failure in the United States. J Card Fail. 2022;28(3):453-466. doi:10.1016/j.cardfail.2021.12.017
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
- Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014-2020). Pharmacoeconomics. 2020;38(11):1219-1236. doi:10.1007/s40273-020-00952-0
- Hessel FP. Overview of the socio-economic consequences of heart failure. Cardiovasc Diagn Ther. 2021;11(1):254-262. doi:10.21037/cdt-20-291
- Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
- Agarwal MA, Fonarow GC, Ziaeian B. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol. 2021;6(8):952-956. doi:10.1001/jamacardio.2020.7472
- Arora S, Patel P, Lahewala S, et al. Etiologies, trends, and predictors of 30-day readmission in patients with heart failure. Am J Cardiol. 2017;119(5):760-769. doi:10.1016/j.amjcard.2016.11.022
- Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355-363. doi:10.1001/jama.2012.216476
- Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429-1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302. doi:10.1056/NEJM199108013250501
- Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100(23):2312-2318. doi:10.1161/01.cir.100.23.2312
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669-677. doi:10.1056/NEJM199209033271001
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342(8875):821-828. doi:10.1016/0140-6736(93)92693-N
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667-1675. doi:10.1056/NEJMoa010713
- Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759-766. doi:10.1016/s0140-6736(03)14282-1
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13. doi:10.1016/S0140-6736(98)11181-9
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007. doi:10.1016/S0140-6736(99)04440-2
- Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199. doi:10.1161/01.cir.0000035653.72855.bf
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi:10.1056/NEJMoa1409077
- Velazquez EJ, Morrow DA, DeVore AD, et al; PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548. doi:10.1056/NEJMoa1812851
- Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94(11):2807-2816. doi:10.1161/01.cir.94.11.2807
- Cleland JGF, Bunting KV, Flather MD, et al; Beta-blockers in Heart Failure Collaborative Group. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26-35. doi:10.1093/eurheartj/ehx564
- Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283(10):1295-1302. doi:10.1001/jama.283.10.1295
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717. doi:10.1056/NEJM199909023411001
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309-1321. doi:10.1056/NEJMoa030207
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21. doi:10.1056/NEJMoa1009492
- Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular
and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190 - McMurray JJV, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi:10.1056/NEJMoa1911303
- Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819-829. doi:10.1016/S0140-6736(20)31824-9
- Solomon SD, McMurray JJV, Claggett B, et al; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089-1098. doi:10.1056/NEJMoa2206286
- Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5(3):178-187. doi:10.1016/s1071-9164(99)90001-5
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in Blacks with heart failure. N Engl J Med. 2004;351(20):2049-2057. doi:10.1056/NEJMoa042934
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314(24):1547-1552. doi:10.1056/NEJM198606123142404
- Khazanie P, Liang L, Curtis LH, et al. Clinical effectiveness of hydralazine-isosorbide dinitrate therapy in patients with heart failure and reduced ejection fraction: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9(2):e002444. doi:10.1161/CIRCHEARTFAILURE.115.002444
- Swedberg K, Komajda M, Bohm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875-885. doi:10.1016/S0140-6736(10)61198-1
- Mann DL, Givertz MM, Vader JM, et al; LIFE Investigators. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol. 2022;7(1):17-25. doi:10.1001/jamacardio.2021.4567
- Mehra MR, Uriel N, Naka Y, et al; MOMENTUM 3 Investigators. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med. 2019;380(17):1618-1627. doi:10.1056/NEJMoa1900486
- Shen L, Jhund PS, Docherty KF, et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur Heart J. 2022;43(27):2573-2587. doi:10.1093/eurheartj/ehac210
- Packer M, McMurray JJV. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23(6):882-894. doi:10.1002/ejhf.2149
- Straw S, McGinlay M, Witte KK. Four pillars of heart failure: contemporary pharmacological therapy for heart failure with reduced ejection fraction. Open Heart. 2021;8(1):e001585. doi:10.1136/openhrt-2021-001585
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1161/CIR.0000000000000509
- Severino P, D’Amato A, Prosperi S, et al; On Behalf Of The Italian National Institute For Cardiovascular Research (INRC). Do the current guidelines for heart failure diagnosis and treatment fit with clinical complexity? J Clin Med. 2022;11(3):857. doi:10.3390/jcm11030857
- Lewis EF. A fourth pillar for all in the treatment of heart failure. Eur Heart J. 2021;42(43):4452-4454. doi:10.1093/eurheartj/ehab612
- Writing Committee, Maddox TM, Januzzi JL Jr, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772-810. doi:10.1016/j.jacc.2020.11.022
- Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365-2383. doi:10.1016/j.jacc.2019.02.015
- Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure-optimizing therapy with the need for speed. JAMA Cardiol. 2021;6(7):743-744. doi:10.1001/jamacardio.2021.0496
- Ghazi L, Yamamoto Y, Riello RJ, et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J Am Coll Cardiol. 2022;79(22):2203-2213. doi:10.1016/j.jacc.2022.03.338
- Clark KAA, Defilippis EM, Morris AA. Breaking the bank: the financial burden of living with heart failure. J Card Fail. 2022;28(9):1434-1436. doi:10.1016/j.cardfail.2022.07.004
- Ali HR, Valero-Elizondo J, Wang SY, et al. Subjective financial hardship from medical bills among patients with heart failure in the United States: the 2014-2018 medical expenditure panel survey. J Card Fail. 2022;28(9):1424-1433. doi:10.1016/j.cardfail.2022.06.009
- Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5(6):e002606. doi:10.1161/JAHA.115.002606
- Ahmad T, Miller PE, McCullough M, et al. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur J Heart Fail. 2019;21(9):1064-1078. doi:10.1002/ejhf.1557
- Teerlink JR, Diaz R, Felker GM, et al; GALACTIC-HF Investigators. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384(2):105-116. doi:10.1056/NEJMoa2025797
- Armstrong PW, Pieske B, Anstrom KJ, et al; VICTORIA Study Group. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883-1893. doi:10.1056/NEJMoa1915928


