- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
FINE-CKD Model to Evaluate Economic Value of Finerenone in Patients With Chronic Kidney Disease and Type 2 Diabetes
ABSTRACT
BACKGROUND: Chronic kidney disease (CKD) is a progressive and irreversible disease often associated with type 2 diabetes (T2D). CKD is associated with an elevated risk of cardiovascular (CV) events, increased mortality, and diminished quality of life. Finerenone is a new treatment for patients with CKD and T2D that delays CKD progression and reduces CV complications.
OBJECTIVE: To describe the approach and structure of a costeffectiveness model for finerenone for patients with CKD and T2D and compare it with existing economic models in CKD.
METHODS: A de novo cost-effectiveness model (FINE-CKD model), reflective of FIDELIO-DKD results, was developed for finerenone. The FINE-CKD model was designed and implemented in accordance with published guidance on modeling and was developed with input from economic and clinical experts. The final model approach was evaluated against existing modeling structures in CKD identified through a systematic literature review.
RESULTS AND CONCLUSIONS: The FINE-CKD model structure follows recommended modeling guidelines and has been designed in accordance with the best practices of modeling in CKD, while also incorporating important features of the FIDELIO-DKD design and results. The approach is consistent with the published literature, ensuring transparency and minimizing uncertainty that can arise from unnecessary complexity. The FINE-CKD model allows for reliable assessment of benefits and costs related to the use of finerenone in patients with CKD and T2D, and it is a reliable assessment of cost-effectiveness.
Am J Manag Care. 2021;27:S375-S382. https://doi.org/10.37765/ajmc.2021.88808
For author information and disclosures, see end of text.
Introduction
The global prevalence of chronic kidney disease (CKD) is approximately 11%, and in 2017, CKD was one of the most common diseases worldwide.1-3 CKD is a progressive and irreversible disease, causing gradual loss of kidney function, thereby increasing the risk of kidney failure (end-stage kidney disease [ESKD]). CKD staging is based on clinical indicators, including estimated glomerular filtration rate (eGFR) stage (G1-G5), and albuminuria level (A1-A3).
In relation to the total population of patients with type 2 diabetes (T2D), patients with concomitant CKD represent approximately 40%. However, the prevalence of CKD in patients with T2D is likely underestimated because many subjects have not received a diagnosis.4,5 In addition to promoting ESKD risk, CKD increases the risk of cardiovascular (CV) events6 and mortality7,8 and reduces quality of life (QOL).9
The current standard of care (SOC) for patients with CKD and T2D includes lifestyle modifications and pharmacotherapy with glucose-lowering and antihypertensive agents, focusing on slowing progression of the disease, preventing ESKD, and reducing the risk of CV events and mortality.10,11 Unfortunately, current treatment modalities are inadequate in achieving these goals, and CKD in T2D remains a condition with high unmet needs.
CKD in patients with T2D is associated with substantial health care costs related to the complex management of the disease and its complications. Along with the deterioration of kidney function and progression to more advanced CKD stages, these patients also tend to experience longer hospital stays and increased medical costs.
Finerenone is a new, selective, nonsteroidal mineralocorticoid receptor (MR) antagonist with higher antagonistic potency for MR and lower relative affinity to other steroid hormone receptors when compared with other MR antagonists.12-14 The FIDELIO-DKD (NCT02540993) trial showed that use of finerenone on top of SOC, compared with SOC alone, provides a reduction in the risk of CKD progression of 18% and a reduction in the risk of CV complications of 14% within a median follow-up of 2.6 years. In addition, finerenone was shown to be well-tolerated with a modest increase in hyperkalemia leading to discontinuation.12-14
In many countries, public funding decisions for interventions such as finerenone are based on health economic evidence, requiring cost-effectiveness models to estimate long-term costs and effects. Accordingly, our objective was to present the structure of the cost-effectiveness model for finerenone and compare it with other economic evaluations in CKD available in the literature. While development of a de novo model for finerenone was deemed necessary to reflect and incorporate the FIDELIO-DKD results fully, a new model should be consistent with best practices of economic modeling in CKD.
FINE-CKD Model
The FINE-CKD model was designed, programmed, and populated with data in accordance with National Institute for Health and Care Excellence recommendations and ISPOR-SMDM guidance on modeling for economic evaluation.15 The development of the model was supported by consultations with economic and clinical experts. The model was implemented in Microsoft Excel 2016, supported by Visual Basic for Applications (VBA).
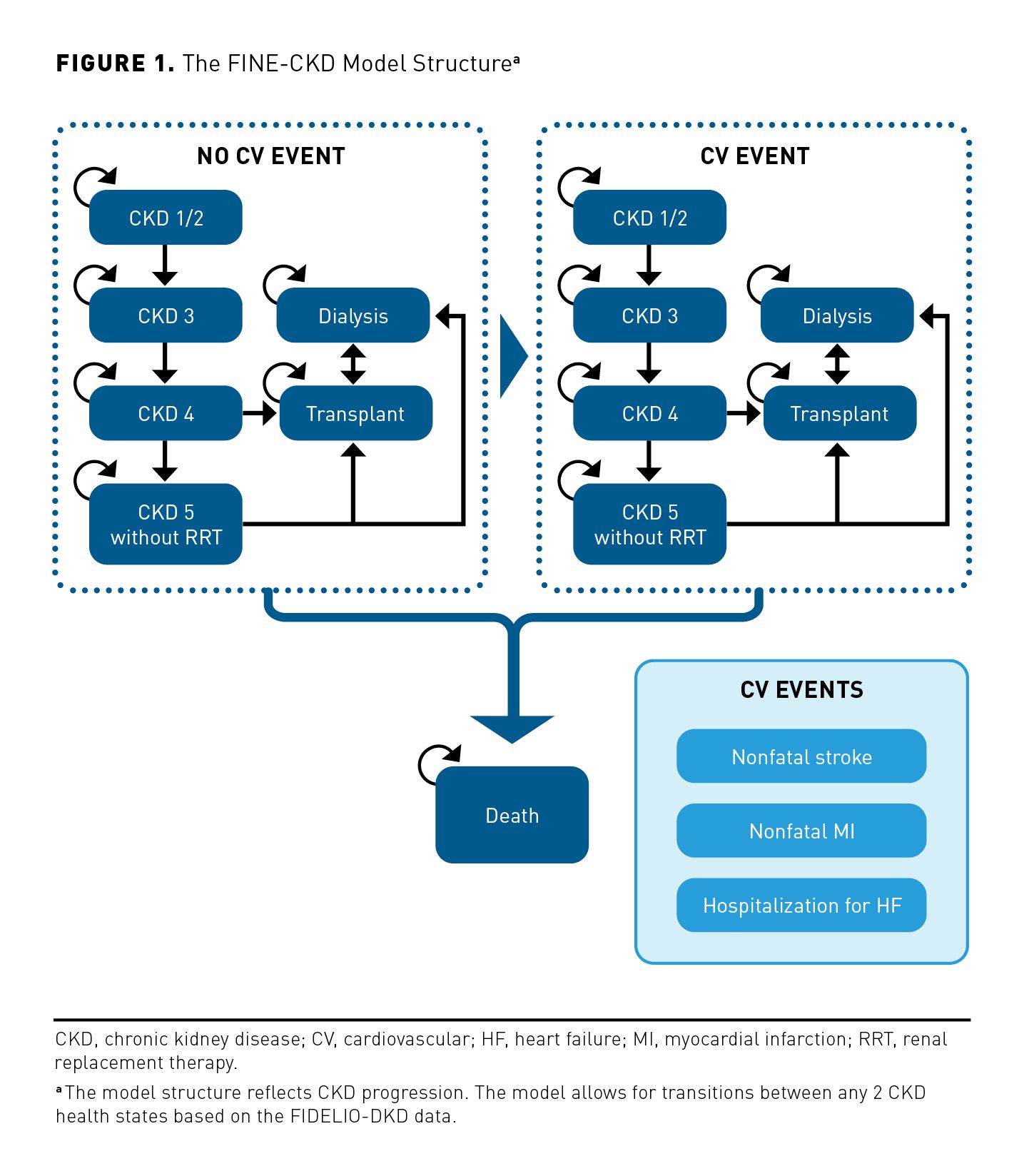
The FINE-CKD model uses a Markov modeling approach with the structure presented in Figure 1. A Markovian approach was deemed an appropriate framework for modeling the relationship between CKD progression and the incidence of CV events because this approach allows for events to occur over time using regular cycles.
To assess cost-effectiveness of finerenone, the FINE-CKD model is based on FIDELIO-DKD, a randomized controlled trial (RCT) that showed that finerenone slows the progression of kidney disease in patients with a clinical diagnosis of CKD and T2D. The secondary outcome of interest in FIDELIO-DKD was the impact of finerenone on the reduction of CV events and death. The finerenone model, therefore, focused on these important dimensions of clinical value (CKD, CV events, and death).
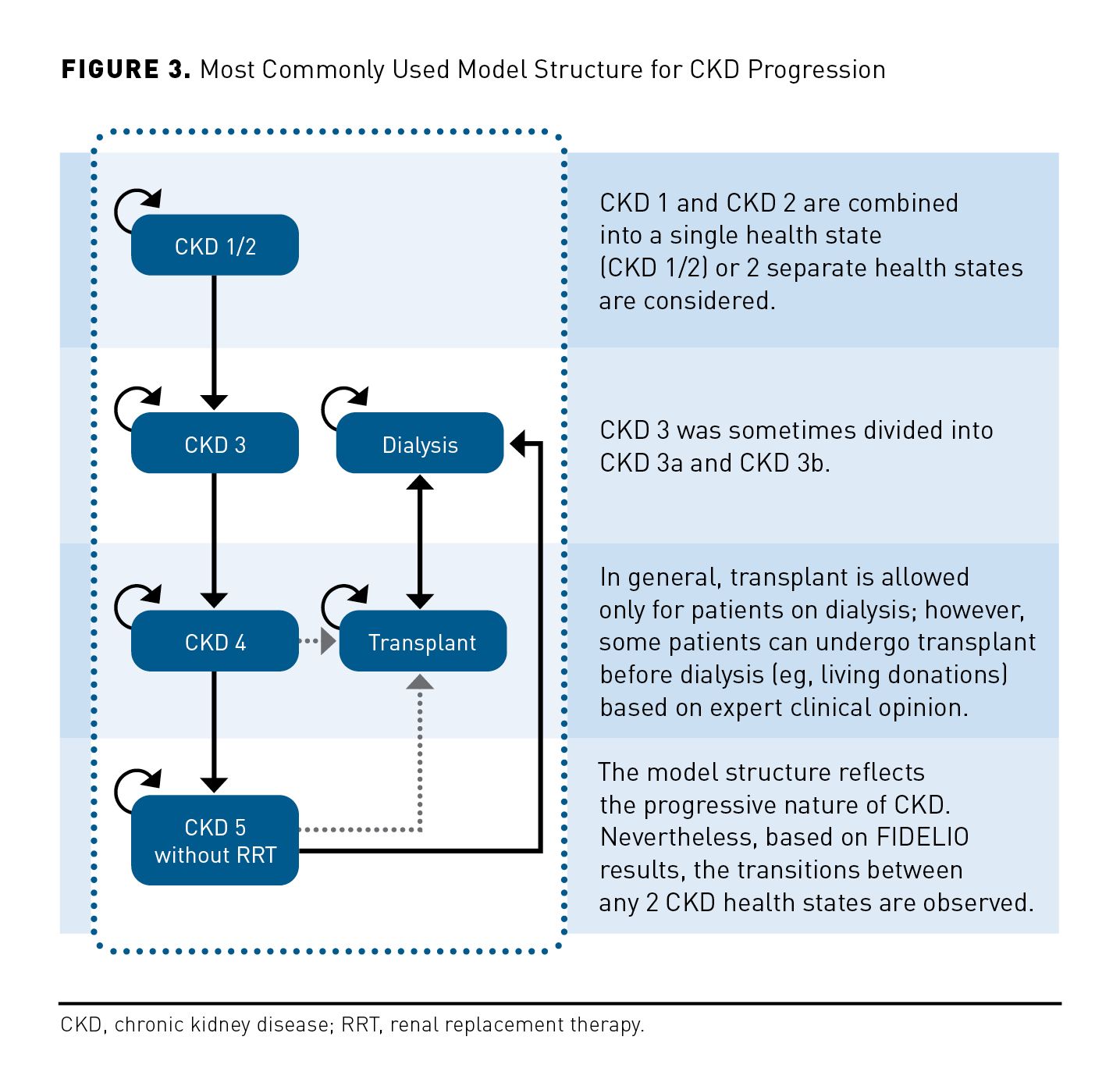
The model health states must represent the main stages in the patients’ disease progression. Similarly, they should reflect the patient’s condition at any time point, as well as the impact of treatment. Therefore, the health states of the FINE-CKD model should represent the key outcomes of FIDELIO-DKD and should be in line with both the potentially important benefits and the safety concerns of finerenone. As such, the model health states were defined according to the stage of kidney disease and history of CV events. Four stages of CKD progression were considered: CKD 1/2, CKD 3, CKD 4, and CKD 5 without renal replacement therapy (RRT), in addition to 2 stages for patients with ESKD (dialysis and transplant). Patients start the model in one of the CKD stages. Patients remain in the same CKD stage, or move to a more/less advanced CKD stage. In each CKD stage patients can experience a modeled CV event (nonfatal myocardial infarction [MI], nonfatal stroke, hospitalization for heart failure [HF]) or death. The model considers 6 corresponding health states for patients after the first CV event observed within the model (ie, CKD 1/2 post CV event, CKD 3 post CV event, CKD 4 post CV event, CKD 5 without RRT post CV event, dialysis post CV event, transplant and CV event). Once patients experience a first CV event, they move to the post CV event health state and are not able to move back to the health state without a history of CV events. Patients can change CKD stage and experience a first CV event in the same 4-month model cycle (ie, a patient from CKD stage 3 can move to CKD stage 4 post CV event). Transitions between all CKD stages are allowed. The assessment of end points occurred every 4 months in the FIDELIO-DKD trial. To reflect the disease progression in alignment with the trial evidence, a 4-month cycle length was used.
In relation to CKD progression, the 2 main predictors for changes in CKD stages are the stage a patient is currently in and the duration they have been in that stage. Transition probabilities between CKD stages in the model, however, depend only on the current stage the patient is in (ie, these transition probabilities are constant over time). The CKD stage can be defined by eGFR and albuminuria level. FIDELIO-DKD included eGFR as a part of the primary outcome, thereby leading to an expectation of the model to estimate the impact of the treatment on changes in this parameter. FIDELIO-DKD provides granular eGFR data, whereas albuminuria was considered only as an explanatory end point. Therefore, the model health states were defined based on the eGFR. Observed changes in eGFR supplemented by subgroup analyses considering albuminuria were deemed sufficient.
In the case of CV events or death, many prognostic factors can be theoretically considered. Nonetheless, there is an established relationship between CKD stage and CV events (fatal or otherwise). As such, the FINE-CKD model focuses on the link between CKD stage and these events rather than extending this to explicit consideration of other risk factors for which no impact of finerenone has been proven.
Generally, patients are permitted within the model to start dialysis after progression to CKD 5, at which point they are eligible for kidney transplant. In practice, because a proportion of patients undergo transplant before dialysis (eg, living donations), the model allows for transplants before dialysis in either CKD stage 4 or CKD stage 5. Additionally, a small percentage of patients undergo dialysis before CKD 4. The model also allows patients to start dialysis again from a transplant state to reflect the risk of graft failure. At any point in the model, patients can die.
Transition probabilities between health states are primarily based on patient-level data from FIDELIO-DKD. Such an approach to modeling CKD progression allows the model to reflect the fluctuations in eGFR over the time. The model is not limited only to considering deterioration of the disease; rather, it reflects the actual trial data. Additionally, data from the literature were applied to estimate long-term outcomes, accounting for increased CV event and mortality risks due to age.
Subsequent CV events are considered in the model within the post-CV event health states. These events are modeled based on the incidence of components of the key secondary end point in FIDELIO-DKD, which did not capture whether CV events were index events or otherwise. The associated costs and disutilities of subsequent CV events were also captured by the model.
The model simulates patients’ trajectories over a lifetime horizon, which is reflective of the chronic nature of CKD in T2D. Patients may die in any health state in the model. In line with the FIDELIO-DKD protocol, different reasons for deaths are accounted for and implemented in the model. These comprise renal death and CV death as well as additional background mortality.
Existing Economic Models in CKD
To design and assess the approach of the FINE-CKD model structure against all relevant cost-effectiveness models available in the public domain, a systematic literature review was conducted. All economic models considering a population of patients with CKD were included, irrespective of the form of economic evaluation used. To consider as broad a population as possible, no restriction on diabetes status was applied. In addition, no restriction was made on interventions, geographical scope, or time frame. The search strategy and PRISMA diagram are presented in eAppendix (eAppendices available at ajmc.com).
The review of literature in selected databases identified a total of 15,147 references after the deduplication process (eAppendix Figure 1, and eAppendix Tables 1 and 2). After screening for titles and abstracts, 14,422 records were excluded. Full texts were then assessed for eligibility, and an additional 670 articles were excluded. The final selection included 55 publications. Additionally, after searching the Health Technology Assessment databases, 6 more reports were included. Finally, 61 studies were extracted. eAppendix Tables 3, 4, and 5 present the main characteristics, including 61 studies.
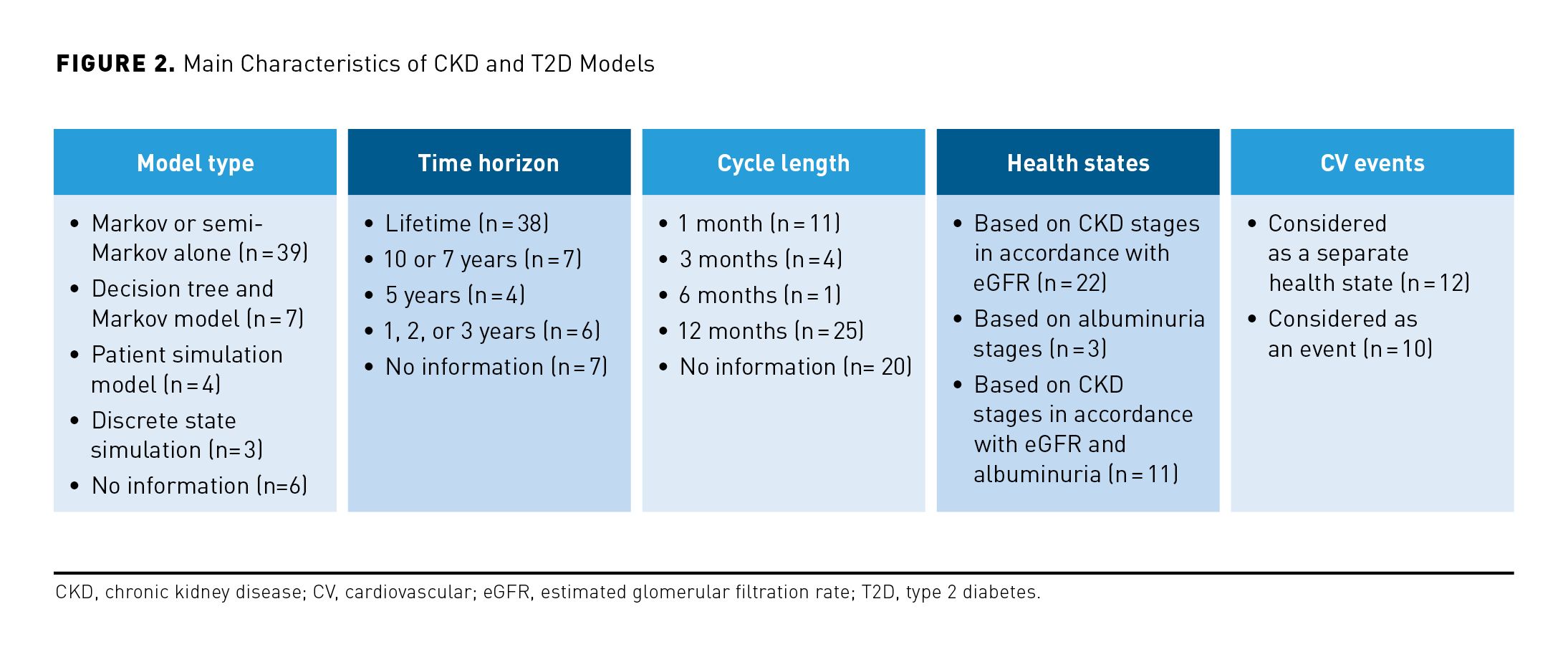
The majority of models applied a lifetime time horizon (n = 38). Generally, published models utilized frameworks based on eGFR and/or albuminuria, with 2 main core structures identified: patient-level models (ie, microsimulation models involving albuminuria and/or eGFR) and cohort-level Markov models, utilizing CKD stages to model CKD progression.
Most models assumed that CKD was progressive and irreversible and included the possibility for patients to experience renal replacement therapy when they reach ESKD in the form of dialysis or transplant. CV complications of CKD were considered in 22 models, including 12 models in which CV events were represented by separate health states. In addition to eGFR, albuminuria was considered as a predictor of CKD progression in some models, explicitly impacting the risk of CV events.
Among the included cost utility analyses and cost-effectiveness analyses (CEAs), there were 39 cohort Markov or semi-Markov models, 7 decision trees together with Markov models, 4 patient-level simulation models, 3 discrete event simulation models, and 6 studies in which the model type was not provided. The remaining 2 publications described cost-benefit analyses.
Based on the analysis of the models identified, the published literature appears to support the use of both a cohort Markov framework and a patient-level microsimulation model. These options are discussed in turn below.
Cohort Markov models
The most common cycle length was 1 year (with the exception of 11 models with a cycle length of 1 month, 4 models with 3-month cycles, and 1 model with 6-month cycles). While variations in structure were evident in the Markov models identified, these models mainly considered the progressive nature of the disease with patients transitioning forward through CKD stages, with most studies not allowing for improvement over time. Health states were based on eGFR in line with KDIGO stratifications (from G1-G5), stage G1 and G2 being sometimes combined, and stage G3 sometimes, although rarely, disaggregated into stages G3a and G3b. These models predicted CKD outcomes using rates of sustained eGFR decline or transition probabilities.
The most common cycle length was 1 year (with the exception of 11 models with a cycle length of 1 month, 4 models with 3-month cycles, and 1 model with 6-month cycles).
Of the models employing a Markov structure, one of the most relevant and recent was an analysis published in 2019 by Go et al to compare CKD screening strategies with no screening. The model was applied to the general Korean population with annual cycles over the lifetime of the cohort.16 The model included 8 CKD-related health states. These were defined in a similar way to FINE-CKD in terms of CKD stage (G1/G2, G3, G4, G5), and there were broad similarities in the way the acute and postacute stages of transplant were distinguished, though Go et al applied a 1-year acute period rather than a 4-month period. Go et al applied more granularity than the FINE-CKD model in terms of both dialysis (distinguishing between hemodialysis and peritoneal dialysis) and CV complications (distinguishing not only between acute and postacute, as in FINE-CKD, but also between MI and stroke). Dialysis and CV complications are aggregated in the FINE-CKD model by applying weighted averages of each component. The incidence of CKD G3 was estimated based on National Health Insurance Scheme claims data, whereas the progression of CKD was assigned based on the literature with the inclusion of relative risk for patients with diabetes. One simplification relative to FINE-CKD is that only the progressive character of CKD was reflected in the model (ie, no improvement was possible). In contrast with the approach taken in FINE-CKD, it was assumed that all CKD G5 patients began RRT in the next cycle. Treatment effects on CKD progression were included by applying a reduction to the rate of transition to more advanced CKD stages. This contrasts with the approach of FINE-CKD, which applies transition probabilities to each arm on the basis of patient-level data from FIDELIO-DKD. The Korean national population-based study was used for obtaining CV risks.
In a UK HTA Assessment Study from 2010, a Markov model was also used to evaluate the cost-effectiveness of early referral strategies in a cohort of patients with CKD who were not known to nephrology services.17 People with diabetes were excluded because these patients receive annual checks for kidney disease in the UK. A lifetime time horizon was applied using annual cycles. Six main health states were considered: G1/G2, G3a, G3b, G4, ESKD, and death. Though the health states are broadly similar to those used in FINE-CKD, CKD stage 3 was disaggregated, and there was less granularity in terms of ESKD and its associated treatment. In addition to transiting unidirectionally through the CKD stages, patients could transit across comorbidity states as they developed microalbuminuria, proteinuria, and CV disease. Although the latter is explicitly accounted for in the structure of FINE-CKD, the impact of albuminuria and proteinuria would be addressed through scenario analysis of subgroups rather than through the structure of the model. CKD progression was simulated using reported average annual rates of eGFR decline rather than patientlevel data observed through a randomized controlled trial, with the level of urine albumin/protein at baseline and calculation of the average proportion of individuals that would be expected to transit annually from each CKD state to the next state also being considered in the UK HTA Assessment Study. Again, trial data were not applied for CV risks as they were in the case of FINE-CKD. Instead, a risk algorithm was used, with adjustments made for sex and hypertension status.
In 2013, Erickson et al published results of a cost-effectiveness analysis of statins for primary prevention of MI and stroke in patients with CKD.18 This Markov model was developed from the US perspective with 3-month cycles over patients’ lifetimes, simulating a population of 65-year-old patients with mild to moderate CKD and moderate hypertension. The model structure, unlike the FINE-CKD structure, did not include health states prior to G3a. CKD progression was based on rates derived from large observational cohorts of comparable patient populations via model calibrations. In contrast with FINE-CKD, only the progressive nature of CKD was included, with no backward transition between CKD stages possible. The modeling of progression in the Erickson et al model differs quite substantially from the FINE-CKD model, with the probability of progression from stages G3a to G3b, G3b to G4, and G4 to G5 being obtained from a separate microsimulation model implemented in SAS software rather than through a Markov model. Additionally, transitions based on a mean annual rate of eGFR decline were estimated from the published observations rather than via actual eGFR scores observed in a single RCT. Similar to FINE-CKD, patients could also experience CV events (limited to MI and stroke), though instead of basing risks on observed trial data, the baseline probabilities of CV events were derived from Framingham risk scores, which were multiplied by CKD stage-specific hazard ratios to reflect the increased risk in more severe CKD stages.
In 2017, Schlackow et al presented a long-term policy model of CV complications in moderate to advanced CKD.19 A Markovian structure was used over lifetime, with annual transitions between a limited range of CKD stages (G3b, G4, and G5) relative to FINE-CKD. This model applied interdependence between renal and CV complications (MI, nonhemorrhagic stroke, or arterial revascularization) using individualized risks estimated from 5 years of follow-up data among almost 10,000 patients with moderate to severe CKD. CKD progression was modeled with transition probabilities using a regression-based approach based on observed transitions between CKD stages during annual periods of follow-up in SHARP study.20 Similar to FINE-CKD, transitions between all CKD stages were captured, including those corresponding to disease improvement. CV events were modeled via parametric proportional hazard multivariate survival models.
Patient-level microsimulation models
Some models adopted more complex methods, such as patient level (n = 4) or discrete event simulations (DES, n = 3). These models were mostly used when considering the treatment and consequences of diabetes, with CKD included as a submodel. CKD outcomes were often predicted from annual rates of eGFR decline, eGFR transition probabilities, and/or albuminuria transition probabilities.
A representative example of a microsimulation model is the ECHO-T2DM model.21 It is a stochastic, multiapplication patient-level model suitable for estimating long-term costs and effects of treatments for T2D. The cycle length was 1 year, and the time horizon was user definable. ECHO-T2DM incorporates key structural features from several well-known T2DM models: the NIH Model, the CORE Diabetes Model, and the Diabetes Decision Analysis and Cost of Type 2 Model, and includes development and progression of key micro- and macrovascular complications and mortality.
The CKD submodule of the ECHO-T2DM model includes both injury (defined by the presence of albuminuria, which is not explicitly considered in the structure of FINE-CKD) and function (described by eGFR, which is considered explicitly in the structure of FINE-CKD). Kidney status is based on KDIGO stages with or without moderately increased or severely increased albuminuria. The functional dimension of CKD is modeled using default values for annual rate of change in eGFR, sourced from another model of CKD,22 which may be modified via user-defined eGFR parameters. As with several other studies described above, the application of annual rate of change differs from the transition probability approach applied to the FINE-CKD model. Macrovascular health states consist of ischemic heart disease, MI, stroke, congestive heart failure (CHF), and peripheral vascular disease. While the components of the composite health state differ, the approach is broadly consistent with the aggregation approach used in the FINE-CKD model. Consistent with FINE-CKD, MI and stroke can occur multiple times within the model (although only once each per cycle), and multiple types of macrovascular events can occur in the same cycle. ECHO-T2DM includes 4 different sets of macrovascular risk equations that can be used in the model, but only 1 set of risk equations can be used in a simulation. None of them, however, are specific for CKD.
Discussion
Several findings from the literature review presented here warrant discussion in the context of the cost-effectiveness model developed for finerenone in the treatment of patients with CKD and T2D (ie, FINE-CKD model).
The identified approaches to model CKD progression all follow the same general model structure whether the population is patients with CKD or patients with diabetes. CKD progression modeling is the most detailed in models focused on CKD patients. In diabetes models, which also include CKD submodels, the structure is similar, but include additional considerations related to diabetes (eg, glycemia) and its complications. As finerenone does not impact T2D progression or glycemia-related parameters, the use of a diabetes model would add unnecessary complexity, while also leading to an oversimplification of CKD progression. Indeed, these models overwhelmingly assume a constant decrease of eGFR, as in the ECHO-T2DM model, which does not reflect natural fluctuations of eGFR over time, as observed in FIDELIO-DKD,13 and lacks granularity. Such an approach potentially results in an imprecise estimation of how eGFR changes through time, which could have important impacts on the accrual of costs and outcomes related to CKD progression.
Our systematic review confirms previous conclusions by Sugrue et al,23 who assert that published models typically utilize frameworks based on either eGFR and/or albuminuria, with 2 core structures identified (ie, microsimulation models and cohort-level Markov models using CKD stages to capture disease progression) and with patient trajectories represented as a series of transitions from one CKD stage to another. Sugrue et al advocate for prediction of individual patients’ eGFR trajectories (ie, patient-level models used to capture the heterogeneity of CKD progression clinical course), which may vary substantially across patients and may be influenced by various risk factors, including CV disease and diabetes. Although such heterogeneity may pose challenges to model CKD progression accurately, a Markovian structure considering these factors has been proven to be sufficient in many of the existing models. Given that a Markov modeling approach at the cohort level is able to capture the key considerations, it is preferred to patient-level simulations, since the latter are more difficult to populate and review.15 Based on these factors, the use of a Markov framework in the FINE-CKD model is justified.
As demonstrated by FIDELIO-DKD,13 finerenone is expected to have a CV benefit, as evident in the observed reduction in the risk of a composite end point of time to first occurrence of CV death, nonfatal MI, nonfatal stroke, or hospitalization for HF by 14% over 2.6 years. Moreover, it was shown in the trial that finerenone delays CKD progression and reduces the risk of renal events, including ESKD. The impact of finerenone on these outcomes can be captured in the FINE-CKD model in an appropriate way due to the inclusion of relevant model health states. These health states are implemented to reflect the main stages in the patients’ disease progression. As the cardiorenal protective properties of finerenone result in delayed progression of CKD and reduced occurrence of CV events, it is important that the FINE-CKD model considers both renal and CV outcomes in the list of health states. As in many identified models, and in line with KDIGO guidelines, the kidney health states were defined according to CKD stage and history of CV events. Indeed, 12 of 22 models consider CV events and include them as separate health states. As in the existing models, different CKD stages from G1 to G5 are considered in the FINE-CKD model. Stage G3 could have been additionally divided into 2 categories; however, this was rarely done in the previous models, and in cases where it was, the disaggregation was most often accompanied by application of the same inputs regarding both resource use and QOL for CKD stages 3a and 3b.
Although adequate modeling of the impact of finerenone on CKD and associated risks is inherently complex, a range of simplifications were applied relative to some of the existing CKD models. Among these, albuminuria is not included explicitly in the model as was the case, for example, in a UK HTA Assessment Study.17 No separate health states for different types of dialysis or CV events are considered in the FINE-CKD model, while both of these were included by Go et al.16 These simplifications have been undertaken in the FINE-CKD model for practical reasons, and their impact on model results can be assessed via sensitivity analysis. At the same time, a detailed application of the results of FIDELIO-DKD is considered in the FINE-CKD model by taking full advantage of their granularity in terms of eGFR changes over time. Indeed, the FINE-CKD model considers a relatively short model cycle and allows for all possible transitions between CKD stages, whereas the focus was solely on the deterioration of the disease in the majority of the existing models.
In conclusion, the FINE-CKD model has been built in accordance with the general practice of modeling in CKD with appropriate focus on the incorporation of the FIDELIO-DKD trial results. Hence, the FINE-CKD model accurately reflects reality in terms of the natural history of CKD while also allowing for reliable assessment of benefits and costs related to the use of finerenone in patients with CKD and T2D. The structure of the FINE-CKD model is that which most adequately addresses the decision problem posed, making the model transparent and reducing uncertainty related to unnecessary complexity. This is, at the same time, the most used and well-validated model type and structure in the published literature, as evident from the systematic literature review described above. Together, these considerations lend substantial weight to the approach of the FINE-CKD model and support its use in assessing the cost-effectiveness of finerenone in patients with CKD and T2D.
Acknowledgments
We would like to acknowledge the technical support provided by Aleksandra Drzewiecka and Monika Palarczyk, from Creativ-Ceutical. D.Z.I.C. is supported by a Department of Medicine, University of Toronto Merit Award and receives support from the CIHR, Diabetes Canada, and the Heart and Stroke Richard Lewar Centre of Excellence.
Author affiliations: University of Toronto, Toronto, ON (DZIC); Bayer AG, Wuppertal, Germany, Berlin, Germany (KF, PM); Université Paris-Dauphine, Paris, France (PL); Creativ-Ceutical, Paris, France, Kraków, Poland (AM, MTP); University of Cambridge, Cambridge, United Kingdom (SM); University of North Carolina Kidney Center and University of North Carolina at Chapel Hill, Chapel Hill, NC (PR-C); University of Washington, Seattle, WA (SDS).
Funding source: This supplement was supported by Bayer AG.
Author disclosures: Dr Cherney has served as a consultant or been on paid advisory boards for Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer Pharmaceuticals, Prometic, Bristol Myers Squibb, Maze, CSL-Behring, and Novo-Nordisk; Dr Cherney has also received grants from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, and Novo-Nordisk. Ms Folkerts and Mr Mernagh have been employed by Bayer AG, sponsor of the study. Ms Folkerts has also held stock or stock options from Bayer AG, sponsor of the study. Mr Mernagh has also previously been an independent consultant for Bayer Pharmaceuticals and has disclosed that the subject matter will be used to support market launch of finerenone. Dr Levy has received honoraria from Bayer Pharmaceuticals for advisory boards related to the development of an economic model for finerenone. Dr Millier and Mr Pochopień have reported no relationships or financial interests with any entity that would pose a conflict of interest with the subject matter of this supplement. Dr Morris has received an honorarium from Bayer Pharmaceuticals for participation in the current manuscript. Dr Roy-Chaudhury has served on consultant/advisory boards for WL Gore; Becton, Dickinson, and Company; Medtronic; Humacyte; Cormedix; Akevia;ViforRelypsa; and Bayer Pharmaceuticals. Dr Sullivan has reported that Bayer Pharmaceuticals supported time to assist with model development.
Authorship information: Concept and design (DZIC, KF, PL, PM, AM, SM, MTP, PR-C, SDS); analysis and interpretation of data (DZIC, KF, PL, PM, AM, SM, MTP, PR-C, SDS); drafting of the manuscript (DZIC, PM, AM, MTP, PR-C); critical revision of the manuscript for important intellectual content (DZIC, KF, PL, PM, SM, PR-C, SDS); statistical analysis (MTP); provision of study materials or patients (KF); administrative, technical, or logistic support (PM); supervision (PM, AM, MTP, PR-C).
Address correspondence to: Michał T. Pochopień, MSc; michal.pochopien@ creativ-ceutical.com
REFERENCES
1. Jager KJ, Kovesdy C, Langham R, Rosenberg M, Zha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96(5):1048-1050. doi:10.1016/j.kint.2019.07.012
2. Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2(2):e000380. doi:10.1136/bmjgh-2017-000380
3. Hill NR, Fatoba ST, Oke JL, et al.. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi:10.1371/journal.pone.0158765
4. Nelson RG, Grams ME, Ballew SH, et al. Development of risk prediction equations for incident chronic kidney disease. JAMA. 2019;322(21):2104-2114. doi:10.1001/jama.2019.17379
5. Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007-2012. BMJ Open Diabetes Res Care. 2016;4(1):e000154. doi:10.1136/bmjdrc-2015-000154
6. Sasso FC, Chiodini P, Carbonara O, et al.. High cardiovascular risk in patients with type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate. The NID-2 Prospective Cohort Study. Nephrol Dial Transplant. 2012;26(6):2269-2274. doi:10.1093/ndt/gfr644
7. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3)(suppl 1):Svii-Sxxii, S1-S772. doi:https://doi.org/10.1053/j.ajkd.2019.01.001
8. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302-308. doi:10.1681/ASN.2012070718
9. Valderrábano F, Jofre R, López-Gómez JM. Quality of life in end-stage renal disease patients. Am J Kidney Dis. 2001;38(3):443-464. doi:10.1053/ajkd.2001.26824
10. American Diabetes Association. Standards of medical care in diabetes-2017 abridged for primary care providers. Clin Diabetes. 2017;35(1):5-26. doi:10.2337/cd16-0067
11. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. Published correction appears in Am J Kidney Dis. 2013;61(6):1049. doi:10.1053/j.ajkd.2012.07.005
12. Bakris GL, Agarwal R, Anker SD, et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):333- 344. doi:10.1159/000503713
13. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229. doi:10.1056/NEJMoa2025845
14. Ruilope LM, Agarwal R, Anker SD, et al. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):345-356. doi:10.1159/000503712
15. Caro JJ, Briggs AH, Siebert U, Kuntz KM, ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--1. Value Health. 2012;15(6):796-803. doi:10.1016/j.jval.2012.06.012
16. Go DS, Kim SH, Park J, Ryu DR, Lee HJ, Jo MW. Cost-utility analysis of the National Health Screening Program for chronic kidney disease in Korea. Nephrology (Carlton). 2019;24(1):56-64. doi:10.1111/nep.13203
17. Black C, Sharma P, Scotland G, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14(21):1-184. doi:10.3310/hta14210
18. Erickson KF, Japa S, Owens DK, Chertow GM, Garber AM, Goldhaber-Fiebert JD. Cost-effectiveness of statins for primary cardiovascular prevention in chronic kidney disease. J Am Coll Cardiol. 2013;61(12):1250-1258. doi:10.1016/j.jacc.2012.12.034
19. Schlackow I, Kent S, Herrington W, et al. A policy model of cardiovascular disease in moderate-toadvanced chronic kidney disease. Heart. 2017;103(23):1880-1890. doi:10.1136/heartjnl-2016-310970
20. Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160(5):785-794e10. doi:10.1016/j.ahj.2010.08.012
21. Willis M, Johansen P, Nilsson A, Asseburg C. Validation of the economic and health outcomes model of type 2 diabetes mellitus (ECHO-T2DM). Pharmacoeconomics. 2017;35(3):375-396. doi:10.1007/ s40273-016-0471-3
22. Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 1. model construction, assumptions, and validation of health consequences. Am J Kidney Dis. 2010;55(3):452-462. doi:10.1053/j. ajkd.2009.11.016
23. Sugrue DM, Ward T, Rai S, McEwan P, van Haalen HGM. Economic modeling of chronic kidney disease: a systematic literature review to inform conceptual model design. Pharmacoeconomics. 2019;37(12):1451- 1468. doi:10.1007/s40273-019-00835-z
24. Evans M, Palaka E, Furuland H, et al. The value of maintaining normokalaemia and enabling RAASi therapy in chronic kidney disease. BMC Nephrol. 2019;20(1):31. doi:10.1186/s12882-019-1228-y
25. Schlackow I, Kent S, Herrington W, et al. Cost-effectiveness of lipid lowering with statins and ezetimibe in chronic kidney disease. Kidney Int. 2019;96(1):170-179. doi:10.1016/j.kint.2019.01.028
26. Campbell JR, Johnston JC, Ronald LA, et al. Screening for latent tuberculosis infection in migrants with CKD: a cost-effectiveness analysis. Am J Kidney Dis. 2019;73(1):39-50. doi:10.1053/j.ajkd.2018.07.014
27. Habbous S, Przech S, Martin J, Garg AX, Sarma S. Cost-effectiveness of first-line sevelamer and lanthanum versus calcium-based binders for hyperphosphatemia of chronic kidney disease. Value Health. 2018;21(3):318-325. doi:10.1016/j.jval.2017.08.3020
28. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: a modeling study. PLoS Med. 2018;15(3):e1002532. doi:10.1371/journal.pmed.1002532
29. Pattanaprateep O, Ingsathit A, McEvoy M, Attia J, Thakkinstian A. Cost-effectiveness analysis of renin-angiotensin aldosterone system blockade in progression of chronic kidney disease. Value Health Reg Issues. 2018;15:155-160. doi:10.1016/j.vhri.2017.12.011
30. de Vries EF, Rabelink TJ, van den Hout WB. Modelling the cost-effectiveness of delaying end-stage renal disease. Nephron. 2016;133(2):89-97. doi:10.1159/000446548
31. Mihaylova B, Schlackow I, Herrington W, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: results of the study of heart and renal protection (SHARP). Am J Kidney Dis. 2016;67(4):576-584. doi:10.1053/j.ajkd.2015.09.020
32. Nguyen HV, Bose S, Finkelstein E. Incremental cost-utility of sevelamer relative to calcium carbonate for treatment of hyperphosphatemia among pre-dialysis chronic kidney disease patients. BMC Nephrol. 2016;17(1):256. doi:10.1186/s12882-016-0256-0
33. Gandjour A, Tschulena U, Steppan S, Gatti E. A simulation model to estimate cost-offsets for a disease-management program for chronic kidney disease. Expert Rev Pharmacoecon Outcomes Res. 2015;15(2):341-347. doi:10.1586/14737167.2015.972375
34. Gros B, Galán A, González-Parra E, et al. Cost effectiveness of lanthanum carbonate in chronic kidney disease patients in Spain before and during dialysis. Health Econ Rev. 2015;5(1):49. doi:10.1186/s13561-015-0049-3
35. Okubo R, Kondo M, Hoshi SL, Yamagata K. Cost-effectiveness of obstructive sleep apnea screening for patients with diabetes or chronic kidney disease. Sleep Breath. 2015;19(3):1081-1092. doi:10.1007/ s11325-015-1134-x
36. Ruggeri M, Bellasi A, Cipriani F, et al. Sevelamer is cost effective versus calcium carbonate for the first-line treatment of hyperphosphatemia in new patients to hemodialysis: a patient-level economic evaluation of the INDEPENDENT-HD study. J Nephrol. 2015;28(5):593-602. doi:10.1007/s40620-014-0122-8
37. You JHS, Ming WK, Lin WA, Tarn YH. Early supplemented low-protein diet restriction for chronic kidney disease patients in Taiwan - a cost-effectiveness analysis. Clin Nephrol. 2015;84(4):189-196. doi:10.5414/CN108560
38. Levy AR, Perkins RM, Johnston KM, et al. An epidemiologic model to project the impact of changes in glomerular filtration rate on quality of life and survival among persons with chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:271-280. doi:10.2147/IJNRD.S58074
39. Mennini FS, Russo S, Marcellusi A, Quintaliani G, Fouque D. Economic effects of treatment of chronic kidney disease with low-protein diet. J Ren Nutr. 2014;24(5):313-321. doi:10.1053/j.jrn.2014.05.003
40. Ruggeri M, Cipriani F, Bellasi A, Russo D, Di Iorio B. Sevelamer is cost-saving vs. calcium carbonate in non-dialysis-dependent CKD patients in Italy: a patient-level cost-effectiveness analysis of the INDEPENDENT study. Blood Purif. 2014;37(4):316-324. doi:10.1159/000365746
41. Adarkwah CC, Gandjour A, Akkerman M, Evers S. To treat or not to treat? Cost-effectiveness of ace inhibitors in non-diabetic advanced renal disease: a Dutch perspective. Kidney Blood Press Res. 2013;37(2-3):168-180. doi:10.1159/000350142
42. Nasution A, Syed Sulaiman SA, Shafie AA. Cost-effectiveness of clinical pharmacy education on infection management among patients with chronic kidney disease in an Indonesian hospital. Value Health Reg Issues. 2013;2(1):43-47. doi:10.1016/j.vhri.2013.02.009
43. Thompson M, Bartko-Winters S, Bernard L, Fenton A, Hutchison C, Di Iorio B. Economic evaluation of sevelamer for the treatment of hyperphosphatemia in chronic kidney disease patients not on dialysis in the United Kingdom. J Med Econ. 2013;16(6):744-755. doi:10.3111/13696998.2013.792267
44. Wiebe N, Klarenbach SW, Chui B, et al. Adding specialized clinics for remote-dwellers with chronic kidney disease: a cost-utility analysis. Clin J Am Soc Nephrol. 2012;7(1):24-34. doi:10.2215/CJN.07350711
45. Hopkins RB, Garg AX, Levin A, et al Cost-effectiveness analysis of a randomized trial comparing care models for chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(6):1248-1257. doi:10.2215/CJN.07180810
46. Orlando LA, Belasco EJ, Patel UD, Matchar DB. The chronic kidney disease model: a general purpose model of disease progression and treatment. BMC Med Inform Decis Mak. 2011;11:41. doi:10.1186/1472- 6947-11-41
47. Vegter S, Tolley K, Keith MS, Postma MJ. Cost-effectiveness of lanthanum carbonate in the treatment of hyperphosphatemia in chronic kidney disease before and during dialysis. Value Health. 2011;14(6):852- 858. doi:10.1016/j.jval.2011.05.005
48. Sabariego C, Grill E, Brach M, Fritschka E, Mahlmeister J, Stucki G. Incremental cost-effectiveness analysis of a multidisciplinary renal education program for patients with chronic renal disease. Disabil Rehabil. 2010;32(5):392-401. doi:10.3109/09638280903171584
49. Scalone L, Borghetti F, Brunori G, et al. Cost-benefit analysis of supplemented very low-protein diet versus dialysis in elderly CKD5 patients. Nephrol Dial Transplant. 2010;25(3):907-913. doi:10.1093/ndt/gfp572
50. Vegter S, Perna A, Hiddema W, et al. Cost-effectiveness of ACE inhibitor therapy to prevent dialysis in nondiabetic nephropathy: influence of the ACE insertion/deletion polymorphism. Pharmacogenet Genomics. 2009;19(9):695-703. doi:10.1097/FPC.0b013e3283307ca0
51. Takahashi T, Reed SD, Schulman KA. Cost-effectiveness of the oral adsorbent AST-120 versus placebo for chronic kidney disease. Nephrology (Carlton). 2008;13(5):419-427. doi:10.1111/j.1440-1797.2008.00960.x
52. Gal-Moscovici A, Sprague SM. Comparative cost-benefit analyses of paricalcitol and calcitriol in stage 4 chronic kidney disease from the perspective of a health plan. Clin Drug Investig. 2007;27(2):105- 113. doi:10.2165/00044011-200727020-00003
53. Van Hout BA, Simeon GP, McDonnell J, Mann JF. Economic evaluation of benazepril in chronic renal insufficiency. Kidney Int Suppl. 1997;51(63):S159-S162.
54. Ludbrook A. A cost-effectiveness analysis of the treatment of chronic renal failure. Appl Econ. 1981;13:337-350.
55. Javanbakht M, Hemami MR, Mashayekhi A, et al. DyeVert PLUS EZ system for preventing contrastinduced acute kidney injury in patients undergoing diagnostic coronary angiography and/or percutaneous coronary intervention: a UK-based cost-utility analysis. Pharmacoecon Open. 2020;4(3):459-472. doi:10.1007/s41669-020-00195-x
56. Kalantar-Zadeh K, Hollenbeak CS, Arguello R, Snyder S, Ashfaq A. The cost-effectiveness of extended-release calcifediol versus paricalcitol for the treatment of secondary hyperparathyroidism in stage 3-4 CKD. J Med Econ. 2020;23(3):308-315. doi:10.1080/13696998.2019.1693385
57. Lokelma. Prescribing information. AstraZeneca; 2020. Accessed October 7, 2021. https://www. scottishmedicines.org.uk/media/5371/sodium-zirconium-cyclosilicate-lokelma-final-august-2020docxfor-website.pdf
58. Patiromer for treating hyperkalaemia. National Institute for Health and Care Excellence. February 13, 2020. Accessed October 7, 2021. https://www.nice.org.uk/guidance/ta623/resources/patiromer-fortreating-hyperkalaemia-pdf-82609015577029
59. Snyder S, Hollenbeak CS, Kalantar-Zadeh K, Gitlin M, Ashfaq A. Cost-effectiveness and estimated health benefits of treating patients with vitamin D in pre-dialysis. Forum Health Econ Policy. 2020;23(1):j/ fhep.2020.23.issue-1/fhep-2019-0020/fhep-2019-0020.xml. doi:10.1515/fhep-2019-0020
60. Widén J, Ivarsson M, Schalin L, et al. Cost-effectiveness analysis of patiromer in combination with renin-angiotensin-aldosterone system inhibitors for chronic kidney disease in Sweden. Pharmacoeconomics. 2020;38(7):747-764. doi:10.1007/s40273-020-00902-w
61. Patiromer for treating hyperkaaemia. National Institute for Health and Care Excellence. February 13, 2020. Accessed October 7, 2021. https://www.nice.org.uk/guidance/ta623
62. Goh BL, Soraya A, Goh A, Ang KL. Cost-effectiveness analysis for the treatment of hyperphosphatemia in predialysis patients: calcium-based versus noncalcium-based phosphate binders. Int J Nephrol. 2018;2018:2138528. doi:10.1155/2018/2138528
63. Patiromer (as patiromer sorbitex calcium) 8.4g and 16.8g powder for oral suspension (Veltassa). AstraZeneca. 2020. Accessed October 7, 2021. https://www.scottishmedicines.org.uk/media/5566/ patiromer-veltassa-resub-final-october-2020-for-website.pdf
64. Elbasha E, Greaves W, Roth D, Nwanko C. Cost-effectiveness of elbasvir/grazoprevir use in treatmentnaive and treatment-experienced patients with hepatitis C virus genotype 1 infection and chronic kidney disease in the United States. J Viral Hepat. 2017;24(4):268-279. doi:10.1111/jvh.12639
65. Tolvaptan for treating autosomal dominant polycystic kidney disease. National Institute for Health and Care Excellence. October 28, 2015. Accessed October 7, 2021. https://www.nice.org.uk/guidance/ta358
66. Tolvaptan 15mg, 30mg, 45mg, 60mg and 90mg tablets (Jinarc). Otsuka Pharmaceuticals (UK) Ltd; 2015. Accessed October 7, 2021. https://www.scottishmedicines.org.uk/media/2416/tolvaptan_jinarc_final_ december_2015_for_website.pdf
67. Adarkwah CC, Gandjour A. Cost-effectiveness of angiotensin-converting enzyme inhibitors in nondiabetic advanced renal disease. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):215-223. doi:10.1586/erp.11.8
68. Clement FM, Klarenbach S, Tonelli M, Wiebe N, Hemmelgarn B, Manns BJ. An economic evaluation of erythropoiesis-stimulating agents in CKD. Am J Kidney Dis. 2010;56(6):1050-1061. doi:10.1053/j. ajkd.2010.07.015
69. Hayashino Y, Fukuhara S, Akizawa T, et al. Cost-effectiveness of administering oral adsorbent AST-120 to patients with diabetes and advance-stage chronic kidney disease. Diabetes Res Clin Pract. 2010;90(2):154-159. doi:10.1016/j.diabres.2010.07.007
70. Nuijten M, Andress DL, Marx SE, Curry AS, Sterz R. Cost effectiveness of paricalcitol versus a nonselective vitamin D receptor activator for secondary hyperparathyroidism in the UK: a chronic kidney disease Markov model. Clin Drug Investig. 2010;30(8):545-557. doi:10.2165/11536310-000000000-00000
71. Nuijten M, Andress DL, Marx SE, Sterz R. Chronic kidney disease Markov model comparing paricalcitol to calcitriol for secondary hyperparathyroidism: a US perspective. Curr Med Res Opin. 2009;25(5):1221- 1234. doi:10.1185/03007990902844097
72. Hogan TJ, Elliott WJ, Seto AH, Bakris GL. Antihypertensive treatment with and without benazepril in patients with chronic renal insufficiency: a US economic evaluation. Pharmacoeconomics. 2002;20(1):37- 47. doi:10.2165/00019053-200220010-00004
73. Ravaghi H, Ebrahimnia M, Farzaneh A, Rostami Z, Madani M. Cost-effectiveness analysis of screening chronic kidney disease in Iran. J Clin Diagn Res. 2019;13(11):IC01-IC04. doi:10.7860/JCDR/2019/42446.13295
74. Critselis E, Vlahou A, Stel VS, Morton RL. Cost-effectiveness of screening type 2 diabetes patients for chronic kidney disease progression with the CKD273 urinary peptide classifier as compared to urinary albumin excretion. Nephrol Dial Transplant. 2018;33(3):441-449. doi:10.1093/ndt/gfx068
75. Ferguson TW, Tangri N, Tan Z, et al. Screening for chronic kidney disease in Canadian indigenous peoples is cost-effective. Kidney Int. 2017;92(1):192-200. doi:10.1016/j.kint.2017.02.022
76. Hoerger TJ, Wittenborn JS, Zhuo X, et al. Cost-effectiveness of screening for microalbuminuria among African Americans. J American Soc Nephrol. 2012;23(12):2035-2041. doi:10.1681/ASN.2012040347
77. Howard K, White S, Salkeld G, et al. Cost-effectiveness of screening and optimal management for diabetes, hypertension, and chronic kidney disease: a modeled analysis. Value Health. 2010;13(2):196- 208. doi:10.1111/j.1524-4733.2009.00668.x
78. Manns B, Hemmelgarn B, Tonelli M, et al. Population based screening for chronic kidney disease: cost effectiveness study. BMJ. 2010;341(7781):c5869. doi:10.1136/bmj.c5869
79. Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290(23):3101-3114. doi:10.1001/jama.290.23.3101

