- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Filling the Gaps in Guideline-Directed Care
Abstract
As American clinicians have tried to reduce heart failure rehospitalizations and improve care for patients with heart failure with reduced ejection fraction (HFrEF), the population of patients who have heart failure with preserved ejection fraction (HFpEF) has emerged as needing attention. Although HFrEF and HFpEF share some characteristics, treatment approaches are different, and treatment options for HFpEF are more limited. All patients would benefit from guideline-directed medical treatment. The FDA has expanded the indications for sacubitril/valsartan to encompass both patients with HFrEF and selected patients with HFpEF, and the sodium-glucose cotransporter-2 inhibitors to reduce heart failure hospitalizations and the risk of cardiovascular death in symptomatic patients with HFrEF. It has also approved vericiguat, an oral soluble guanylyl cyclase activator. In addition, investigators are examining possible uses of omecamtiv mecarbil and nonsteroidal aldosterone antagonists in heart failure. Addressing heart failure is a team effort, and such teams need overlapping expertise, innovative approaches, and resources that support and sustain their efforts. Team members should familiarize themselves with the American College of Cardiology 2021 Update to the 2017 Expert Consensus Decision Pathway as a means to offer the best care to the patients that they serve.
Am J Manag Care. 2021;27(suppl 9):S183-S190. https://doi.org/10.37765/ajmc.2021.88672
Introduction and Heart Failure Overview
Nationally, the medical community continues to focus on decreasing readmission rates for heart failure (HF). HF affected an estimated 6 million American adults in 2018. Its prevalence increases significantly with ages older than 80 years.1 Cardiologists categorize HF into primarily 2 groups: HF with reduced ejection fraction (HFrEF, formerly called systolic HF) and HF with preserved ejection fraction (HFpEF, formerly called diastolic HF). HFrEF indicates that a patient’s left ventricular ejection fraction (LVEF)—a marker of underlying pathophysiology—is less than 40% on echocardiography. HFrEF has traditionally been the most well-recognized type of HF.2-5
HFpEF refers to patients with HF symptoms, impaired left ventricular relaxation, probable increased left ventricular chamber stiffness, and an LVEF greater than 50% on echocardiography.6 Patients with HFpEF tend to be older than those with HFrEF. They often have underlying comorbidities of hyperlipidemia, diabetes, atrial fibrillation, anemia, and chronic obstructive pulmonary disease. Patients with HFpEF often experience diastolic dysfunction, reduced compliance (ease of filling a chamber of the heart with blood), ventricular hypokinesia, and a triad of symptoms including shortness of breath, excessive fatigue, and exercise intolerance.7 Among patients with HF who are hospitalized, statisticians indicate that approximately half have reduced ejection fraction (EF) and the other half have preserved EF.2,5
The astute reader will have identified a gap between the LVEF cutoff numbers for HFrEF and HFpEF. The 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized with Heart Failure acknowledges this group, labeling them patients with midrange ejection fraction (HFmrEF, LVEF 40 to 50). In the newly issued Universal Definition and Classification of Heart Failure; A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure (Universal Definition and Classification of Heart Failure), this group is called HF with mildly reduced EF (HFmrEF).5 Management draws on components of both HFrEF and HFpEF.2
HF’s prevalence is expected to increase 46% before 2030, resulting in more than 8 million Americans with HF.1 According to the Atherosclerosis Risk in Communities (ARIC) study, a prospective epidemiologic study conducted in 4 US communities’ surveillance data, women experience slightly higher new-onset HF than men, with Black patients experiencing greater numbers of hospitalizations. HFpEF is more common in women, while HFrEF is common in men.7,8 ARIC surveillance data from 2005 through 2014 indicated that 11.6 people were hospitalized for HF for every 1000 residents. It also showed increased rates of HF hospitalizations, primarily driven by HFpEF, thus making HFpEF an emerging concern.8
Diagnosis and Its Challenges
HF, a progressive condition, begins with risk factors for cardiac dysfunction and proceeds to asymptomatic changes in cardiac structure and function. It evolves into clinical HF, disability, and death. Clinicians use 2 grading systems to classify patients. First, the recently published Universal Definition and Classification of Heart Failure proposes classifying HF based on its progressive pathophysiology5:
- Stage A: At-risk for heart failure
- Stage B: Pre-heart failure
- Stage C: Heart failure
- Stage D: Advanced heart failure
Treatment goals differ based on the stage of the patient’s HF.9 In stage A, patients are at risk for, but do not have, HF and goals are to establish a heart healthy lifestyle, prevent vascular and coronary disease, and prevent left ventricular structural abnormalities. These patients have risk factors for HF (eg, atherosclerotic disease, diabetes, hypertension, metabolic syndrome, obesity). At stage D, the goals are palliative: to control symptoms, improve health-related quality of life, reduce hospital readmissions, and establish patients’ end-of-life goals. The American Heart Association posts a flow chart that concisely summarizes the stages (which have not been changed to the consensus statement’s terminology, but are similar), patient profiles, and recommended therapies at www.heart.org/-/media/files/health-topics/heart-failure/rahf-guidelines-toolkit-algorithm.pdf?la=en.9
Data from various sources indicate survival after onset of HF in older adults has improved. But improvements in HF survival have not been level across all demographics. In the ARIC study, the 30-day, 1-year, and 5-year case fatality rates after hospitalizations were 10.4%, 22%, and 42.3%, respectively. Five-year case fatality rates were higher in Black patients than White patients.10 Among Medicare beneficiaries, the overall 1-year HF mortality rate declined slightly from 1998 to 2008; however, the rate still approached 30%.11
Patients with HFrEF may present with a variety of symptoms, but most are nonspecific.12 Typical symptoms include dyspnea, orthopnea, paroxysmal nocturnal dyspnea, fatigue, and dependent edema. Abdominal bloating, right upper-quadrant discomfort, and early satiety—all nonspecific symptoms—may indicate right-sided HF. Clinicians use a second classification system, the New York Heart Association’s (NYHA) functional classification (see Table 113), to grade symptom severity. Specific signs of HF include elevated jugular venous pressure, positive abdominojugular reflux, S3 (gallop rhythm), and laterally displaced apical impulse; these symptoms indicate a higher degree of congestion (volume overload) and higher risk of hospitalization or death.12,14
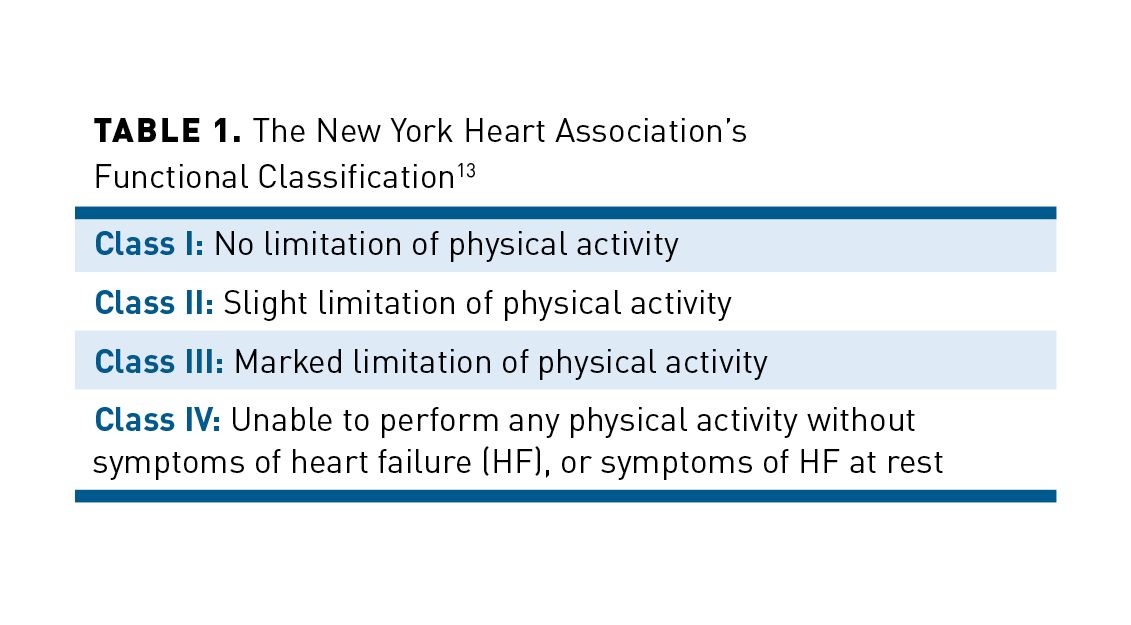
Assessment of a chest x-ray, electrocardiogram, and B-type natriuretic peptide/N-terminal pro–B-type natriuretic peptide (BNP/NT-proBNP) levels are appropriate in patients presenting with HF symptoms. Natriuretic peptide levels can exclude or support an HF diagnosis and predict the patient’s prognostic trajectory. In patients with chronic HFrEF, a decrease in NT-proBNP to 1000 pg/mL or less during treatment has been linked to lower risk of HF hospitalization or cardiovascular (CV) death. The diagnosis should then be confirmed via transthoracic echocardiography.12
Treatment of HFrEF: Standard of Care
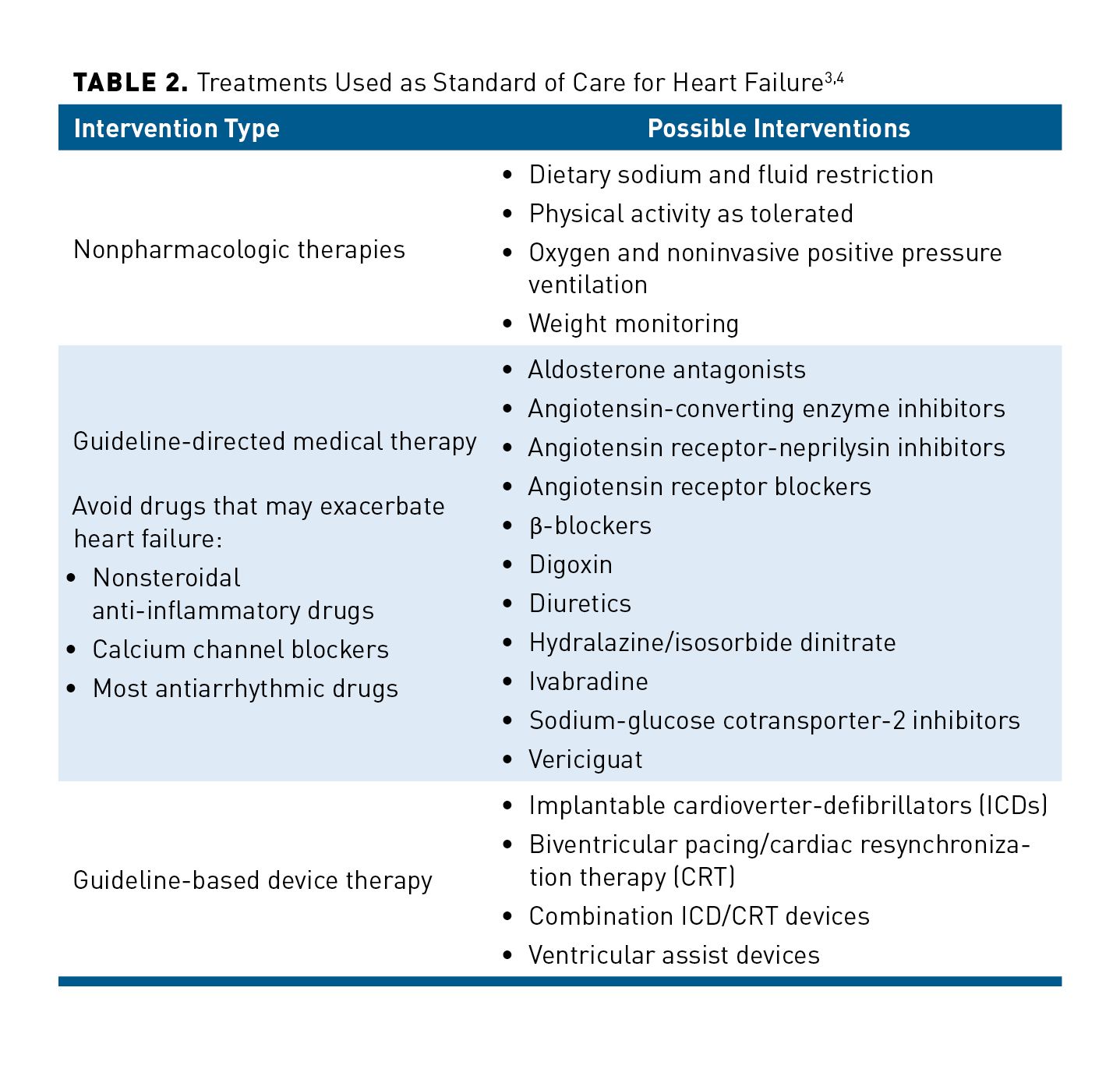
HF is usually associated with multiple comorbidities, necessitating the use of multiple medications. Table 23,4 summarizes the standard of care for HFrEF, listing nonpharmacologic, pharmacologic, and invasive strategies depending on the severity of the patient’s disease.3,4,15-17 Various guidelines detail what to use first based on the patient’s classification or functional status.
Guideline-directed Medical Therapy Advancements
Sacubitril/valsartan
Several vasoactive peptides (eg, adrenomedullin, bradykinin, natriuretic peptides, and substance P) are key to HF’s pathogenesis and progression. Neprilysin, also called neutral endopeptidase, is a metalloprotease that inactivates many vasoactive peptides.18 Clinicians need to be aware that angiotensin II is a neprilysin substrate, and neprilysin inhibitors raise angiotensin levels. For this reason, guidelines indicate neprilysin inhibitors must be administered with an angiotensin receptor blocker (ARB) and not given with angiotensin-converting enzyme inhibitors (ACEIs), as coadministration increases risk of angioedema.19 Angiotensin receptor-neprilysin inhibitors (ARNIs) have been associated with improvement in diastolic function, left ventricular function, quality of life, and burden of ventricular arrhythmias. Currently, one angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, is available.
The randomized controlled prospective comparison of ARNI versus ACEI, PARADIGM HF, study examined sacubitril/valsartan in patients with chronic HFrEF. The investigators enrolled patients with NYHA class II to IV symptoms with an EF less than 40% (but reduced it to less than 35%, 1 year into the trial). Participants had to have stable ACEI or ARB doses and be taking other background guideline-directed medical therapy (GDMT). Participants could not have histories of angioedema, eGFR less than 30 mL/min/1.73m2, symptomatic hypotension or systolic blood pressure (SBP) less than 100 mm Hg, or current decompensated HF.20
In participants treated with sacubitril/valsartan, the primary outcomes of CV death or HF hospitalization had an absolute risk reduction of 4.7% (hazard ratio (HR), 0.80; 95% CI, 0.73-0.87; P <.001) when compared with enalapril-treated patients. The number needed to treat to prevent 1 CV death or HF hospitalization over 27 months was 21. Also, each individual outcome within the composite end point including CV death (HR, 0.80; 95% CI, 0.71-0.89; P <.001) and hospitalization for worsening HF (HR, 0.79; 95% CI, 0.71-0.89; P <.001), as well as the secondary end point of death from any cause (HR, 0.84; 95% CI, 0.76-0.93; P <.001), met statistical significance. Sacubitril/valsartan-treated patients were more likely to experience symptomatic hypotension (14.0% vs 9.2%; P <.001), but the investigators did not identify worsening renal function. Angioedema was similar in both arms.20
The 2016 European Society of Cardiology Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure recommended an ARNI, ACEI, or ARB to reduce morbidity and mortality in patients with chronic HFrEF (with ARNIs preferred).16 They also recommended transitioning patients with NYHA class II to III symptoms who tolerated an ACEI or ARB to an ARNI, basing the recommendation on reduced morbidity and mortality.16 The 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction (2021 ECDP) indicates that clinical studies and prescribers’ clinical experience suggest that initiating an ARNI without previous patient exposure to an ACEI or ARB is safe and effective.3 Common adverse reactions includehypotension, hyperkalemia, cough, dizziness, and renal failure.21
Clinicians had historically started patients on neprilysin inhibitors while they were community dwelling. TheComparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute HF Episode (PIONEER-HF) trial found that clinicians in hospital settings could safely start patients hospitalized for acute decompensated HF on an ARNI as well. This multicenter trial enrolled patients hospitalized for HFrEF from day 2 until day 10 following initial presentation and evaluated the time-averaged reduction in NT-proBNP concentration from baseline through weeks 4 and 8. Prior to randomization, patients were required to have an SBP greater than or equal to 100 mm Hg in the past 6 hours and no intravenous (IV) inotropes in the past 24 hours. Patients received either sacubitril/valsartan or enalapril twice daily with initial dosing adjusted according to SBP. Patients with an SBP between 100 and 119 mm Hg were initiated on sacubitril/valsartan 24/26 mg twice daily or enalapril 2.5 mg twice daily. Patients with an SBP greater than or equal to 120 mm Hg were started on sacubitril/valsartan 49/51 mg twice daily or enalapril 5 mg twice daily. Patients who received sacubitril/valsartan had a significantly greater reduction in time-averaged NT-proBNP concentration when compared with enalapril (-46.7% vs -25.3%; ratio of change, 0.71; 95% CI, 0.63-0.81; P <.001). In addition, the rates of worsening renal function, hyperkalemia, angioedema, and symptomatic hypotension did not differ significantly between the groups within this trial.22 Since symptomatic hypotension was the only adverse effect that occurred with great frequency within a previous trial, the investigators’ decision to maintain the lowest dose of sacubitril/valsartan (24/26 mg twice daily) in patients with an SBP from 100 to 119 mm Hg appears to have mitigated this risk.20,22
Sodium-Glucose Cotransporter-2 Inhibitors
The sodium-glucose cotransporter-2 (SGLT2) inhibitors have recently emerged as a novel therapy in the care of patients with HF. SGLTs block reabsorption of filtered glucose in the proximal renal tubule causing urinary glucose excretion. After the publication of the 2017 ACC Expert Consensus Decision Pathway, the FDA approved SGLT2 inhibitors for treatment of HFrEF patients.3 Results from 2 pivotal studies, DAPA-HF and EMPEROR-Reduced, showed that SGLT2 inhibitors exhibit a beneficial class effect in patients with HFrEF.23 The DAPA-HF study and the EMPEROR-Reduced demonstrated SGLT2 inhibitors’ utility in HF.24,25
The DAPA-HF trial followed randomly assigned patients with chronic HFrEF (N = 4744) treated with dapagliflozin or placebo for an average of 18.2 months. All patients received appropriate neurohormonal antagonists as background treatments. More than 90% received renin-angiotensin system inhibitors and β-blockers, and more than 70% took mineralocorticoid receptor antagonists. Approximately half of the patients had diabetes at baseline, and more than 40% did not have underlying ischemic heart disease.24
In this patient population, the composite outcome of CV death, hospitalization for HF, or an urgent visit for worsening HF requiring IV therapy occurred in 16.3% of patients taking dapagliflozin and 21.2% of patients taking placebo (HR, 0.74; 95% CI, 0.65-0.85; P <.001). In addition, each efficacy outcome within the composite occurred statistically significantly less often in the population receiving dapagliflozin versus placebo. The composite outcome occurred with similar regularity in patients with diabetes (HR, 0.75; 95% CI, 0.63-0.90) as without diabetes (HR, 0.73; 95% CI, 0.60-0.88).24 Subsequent to DAPA-HF, Jhund et al reevaluated the trial data to determine if dapagliflozin also had appreciable effects on recurrent events within the composite end point as opposed to first events. Using the Lin Wei Yang Ying model to determine the rate of recurrent HF hospitalizations and CV deaths, the authors found that the rate ratio of dapagliflozin was statistically significant (HR, 0.75; 95% CI, 0.65-0.88; P <.0002). This showed that the effect of dapagliflozin in patients with HFrEF did not simply diminish first events, but also reduced subsequent events as well.26
The double-blind, placebo-controlled EMPEROR-Reduced trial randomized 3730 patients with class II to IV HFrEF with or without diabetes to receive 10 mg daily or placebo. Patients in this trial also received GDMT as background.25 The investigators followed the participants for a median of 16 months. Empagliflozin reduced the primary end point, a composite of CV death or HF hospitalization significantly (HR, 0.75; 95% CI, 0.65-0.86; P <.001). The event rates were 15.8 and 21/100 patient-years in the empagliflozin and placebo groups, respectively. This translated to a number needed to treat of 19, which the investigators attributed to a lower risk of HF hospitalization. Empagliflozin-treated patients also experienced a significantly slower annual rate of decline in eGFR (a secondary outcome) and lower risk of serious renal outcomes.25
Empagliflozin’s benefits were similar among patients receiving currently recommended drugs, including an ARNI whether or not they had diabetes. Adverse effects associated with HF drugs were similar in both arms, but patients in the SGLT2 inhibition arm were more likely to develop uncomplicated urinary tract infections.25
The 2021 ECDP includes an update on when to add, switch, and titrate all HFrEF patients to maximally tolerated and ideal target doses of SGLT2 inhibitors.3 It recommends initiating patients with stage C HFrEF with ARNI/ACEI/ARB (ARNI preferred) and an evidence-based β-blocker. Additionally, diuretics should be used as needed. Selected patients who meet specific criteria— HFrEF (EF at or below 40%) with or without diabetes and NYHA class II to IV—are treated with SGLT2 inhibitors as additional secondary agents.3
Among patients with HF, SGLT2 inhibitors have been well tolerated. The FDA-approved product labeling indicates that common adverse effects include genital yeast infections, including vulvovaginal candidiasis and balanitis, frequent urination, and urinary tract infection.27-29 Canagliflozin, dapagliflozin, and empagliflozin should be discontinued at least 3 days before scheduled surgery.27-29 SGLT2 inhibitors are dosed once daily. They have few drug interactions, but canagliflozin is a P-glycoprotein substrate.27 SGLT2 inhibitors are contraindicated in patients with type 1 diabetes, patients who are lactating, and those on dialysis.27-29 Dapagliflozin may be prescribed in patients with eGFR at or above 30 mL/min/1.73m2 and empagliflozin may be prescribed in patients with eGFR at or above 20 mL/min/1.73m2.28,29
In the aforementioned studies, infections were common during early treatment.23 Adverse effects (AEs) in HF trials in which many patients did not have diabetes were less severe than in trials enrolling patients with diabetes. In DAPA-HF, discontinuation due to serious AEs was similar between arms. Other serious AEs included volume depletion (1.2% and 1.7%, P = .23) and renal AEs (1.6% and 2.7%) in the dapagliflozin and placebo groups, respectively. Dapagliflozin-treated patients experienced no notable excess AEs.24 In EMPEROR-REDUCED, uncomplicated genital tract infection was reported more frequently in the empagliflozin group. AEs identified often in previous trials including hypotension, volume depletion, renal dysfunction, bradycardia, and hyperkalemia were not identified as concerns in this trial. 25
The ECDP warns that patients may be at risk for genital mycotic infections, ketoacidosis in patients with diabetes, acute kidney injury, urosepsis, pyelonephritis, and Fournier gangrene. SGLT2 inhibitors should also not be prescribed to women who are pregnant or breastfeeding. Patients who develop acute kidney injury from treatment with SGLT2s may need to be held until the eGFR improves to above 30 mL/min/1.73m2 with dapagliflozin and above 20 mL/min/1.73m2 with empagliflozin. Because SGLT2 inhibitors may enhance volume depletion, dose reduction of loop diuretics may be warranted dependent on the volume status of the patients being treated.3
Vericiguat
The FDA approved vericiguat, an oral soluble guanylyl cyclase activator, in January 2021 for patients with HFrEF with worsening disease to reduce risk of CV death and HF hospitalization, following a hospitalization for HF, or need for outpatient IV diuretics, in adults with symptomatic chronic HF and EF less than 45%.30,31
Activation of guanylyl cyclase increases cGMP, which leads to natriuresis, diuresis, and vasodilation. In the VICTORIA randomized double-blind placebo-controlled trial, patients were given vericiguat titrated to a 10-mg target dose or placebo in addition to GDMT.32 Enrolled patients had symptomatic chronic HF (NYHA class II, III, or IV) and an EF of 45% or less within the previous 12 months. They also had elevated natriuretic peptide levels within 1 month of randomization. Patients were excluded if they had an SBP less than 100 mm Hg, had an implantable left ventricular assist device, or were on concomitant long-acting nitrates, soluble guanylate cyclase stimulators (eg, riociguat), phosphodiesterase type 5 inhibitors (eg, sildenafil), or IV inotropes. In addition, enrollment required objective evidence of worsening HF in the form of a hospitalization within the past 3 to 6 months or the use of IV diuretics without hospitalization. In the vericiguat arm, 35.5% of participants experienced the primary outcome (a composite of death from CV causes or first hospitalization for HF) compared with 38.5% of the placebo arm (HR, 0.90; 95% CI, 0.82-0.98; P = .02). Secondary outcomes included CV deaths (16.4% vs 17.5% [HR, 0.93; 95% CI, 0.81-1.06]), all-cause death (20.3% vs 21.2%), HF hospitalization (27.4% vs 29.6% [HR, 0.90; 95% CI, 0.83-0.98; P = .02]), and serious AE (32.8% vs 34.8% in vericiguat-treated participants and placebo-treated participants, respectively). The positive composite end point was primarily driven by improvements in HF hospitalizations, but the trial’s 1-month duration may have diminished the authors’ ability to find a difference in deaths from CV causes. Hypotension (9.1%) and anemia (7.6% and 5.7%) in the vericiguat and placebo groups, respectively, were common AEs.32
Vericiguat could be considered as an adjunct therapy to reduce the risk of HF hospitalization in symptomatic patients with objective evidence of worsening HF. This could include patients who receive outpatient IV diuretics as a means to prevent worsening HF and subsequent hospitalization.
HFpEF: Many Unmet Needs
Few randomized, controlled studies have shown clinical benefits in the use of medications to treat HFpEF, substantiating experts’ designation of this condition as having many unmet needs. HFpEF is a heterogeneous condition, although some cardiologists divide it broadly into 3 groups: exercise-induced elevation of left ventricular filling pressures (lowest risk, difficult to diagnose), volume overload (more common, easier to recognize), or right HF (highest risk, poor prognosis).22,33 Regardless of HFpEF’s cause or presentation, clinicians and patients need to treat/monitor blood pressure and volume and address modifiable risk factors aggressively.4,17
There are many similarities and differences among patients with HFpEF and HFrEF. Smoking increases risk of both HFpEF and HFrEF, and both are associated with accelerated cognitive decline and an equal likelihood of rehospitalization. Physical activity lowers risk of developing HFpEF, but not HFrEF. Atrial fibrillation is marginally more prevalent in patients with HFpEF (HR, 2.34; 95% CI, 1.48-3.70) than those with HFrEF (HR, 1.32; 95% CI, 0.83-2.10). Patients with HFpEF are more likely to be women, obese, anemic, and have hypertension.1
Clinicians should approach HFpEF differently than they do HFrEF.33 Although β-blockers, ACEIs, and cardiac resynchronization are effective in HFrEF, in HFpEF these interventions do not lead to statistically significant improvements in morbidity and to date no therapies have been shown to improve mortality in HFpEF. Patients with HFpEF have no or minimal left ventricular dilatation, yet therapies that improve mortality in HFrEF also reverse its associated left ventricular dilatation. The 2013 ACCF/AHA Guideline for the Management of Heart recommended several approaches to manage HFpEF that remained largely unchanged in the 2017 update3,4:
Control blood pressure
Use diuretics to relieve symptoms due to volume overload
Manage atrial fibrillation according to published guidelines
Using ACEIs, ARBs, and β-blockers is reasonable to control blood pressure
Aldosterone antagonists can be considered to reduce hospitalizations in patients with an EF ≥45%, elevated BNP levels, or HF admission within a year
The use of ARBs might be considered to reduce hospitalizations
The 2017 Focused Update included an update to only one treatment recommendation: the use of aldosterone antagonists. The Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist (TOPCAT) trial initially showed that spironolactone did not reduce the composite end point of CV death, aborted cardiac arrest, or hospitalization for the management of HF at its conclusion and initial publication.34 However, the investigators found considerable regional variability among the trial participants. Therefore, they conducted a post hoc analysis and found a 4-fold difference in the incidence of the primary end point in the Americas (HR, 0.83) when compared with Russia/Georgia (HR, 1.10), which led to the guideline change listed above.3,4
In February 2021, the FDA approved an expanded indication for sacubitril/valsartan.21 This was due to the findings of the Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction (PARAGON-HF) trial. PARAGON-HF was a randomized, double-blind, active-comparator trial. This trial enrolled 4822 patients aged 50 years or older with symptomatic HF, an EF at or exceeding 45% in the past 6 months, elevated natriuretic peptides, evidence of structural heart disease, and background diuretic therapy. The investigators randomized participants to sacubitril/valsartan titrated to a target dose of 97/103 mg twice daily or valsartan titrated to a target dose of 160 mg twice daily. The primary outcome was a composite of total HF hospitalizations and CV death over roughly 3 years. Within the sacubitril/valsartan group, 894 events occurred; 1009 events occurred within the valsartan group (rate ratio, 0.87; 95% CI, 0.75-1.01; P = .06). Therefore, sacubitril/valsartan did not significantly lower the incidence of the primary end point. However, among the subgroup of patients with an EF between 45% and 57%, there was a significant improvement in the primary end point (rate ratio, 0.78; CI, 0.64-0.95), which led the FDA to approve the expanded indication.35
To further evaluate the overall efficacy of sacubitril/valsartan in relationship to a patient’s underlying LVEF, Solomon et al conducted a pooled analysis of patients enrolled within the PARADIGM-HF and PARAGON-HF trials. Patients were divided into groups by LVEF less than or equal to 22.5%, greater than 22.5% to 32.5%, greater than 32.5% to 42.5%, greater than 42.5% to 52.5%, greater than 52.5% to 62.5%, and greater than 62.5% to determine if there were any differences in event rates for first HF hospitalization or CV death, HF hospitalization, CV death, noncardiovascular death, and all-cause mortality. The investigators found variability in the event rates among patients in the prespecified LVEF groups treated with sacubitril/valsartan compared with an ACEI or ARB. The patients with the lowest LVEFs had greatest reduction in clinical end points (LVEF <42.5%). However, all end points failed to meet statistical significance once the LVEF was above or at 42.5%. This was most consistent in the primary composite end point of first hospitalization and CV death (LVEF ≤22.5%: HR, 0.77; 95% CI, 0.63-0.94; P = .012; LVEF ≥22.5% to 32.5%: HR, 0.81; 95% CI, 0.71-0.92; P = .001; LVEF >32.5% to 42.5%: HR, 0.81; 95% CI, 0.69-0.94; P = .005). This analysis showed the benefits of sacubitril/valsartan when compared with ACEIs or ARBs varied by LVEF, but that the benefits did occur in patients with HFmrEF.36
The labeling for sacubitril/valsartan now indicates it can be used to reduce the risk of CV death and HF hospitalization in adult patients with chronic HF. It acknowledges that patients with LVEF below normal have benefited most. This expands the population of patients with HF to include some whose EF is preserved. The labeling also states LVEF is a variable measure and clinical judgment should be used in deciding whom to treat.21
Reaching Beyond Standard of Care
With so many medications, cardiac devices, surgical options, and lifestyle adaptations, patient-centered HF care is complicated. Educating, monitoring, and engaging patients who have HF takes a village of healthcare providers, many of whom are specialists. The average patient’s advancing age and comorbid cardiac and noncardiac conditions also pose problems (more than half of Medicare beneficiaries with HF have 4 or more noncardiovascular comorbidities and more than 25% have 6 or more).37 Finding approaches that are effective and, more importantly, able to be tolerated or lack drug interactions with patients’ critical medications requires a skilled medical team. In addition, care delivery inefficiencies and misunderstandings further increase risk for suboptimal care.3
Streamlined transitions of care have been challenging for many facilities. One innovative program developed to improve transitions and 30-day readmission rates engaged a transitions-of-care pharmacist, community paramedics, and advanced care practitioners.38 Because patients with HF use emergency department services frequently, community paramedics were assigned to make home visits; during those visits they conducted physical assessments, provided disease and medication education, and administered in-home IV medication. Paramedics worked closely with the transitions-of-care pharmacist and advanced care practitioners. Over 7 months, the readmission rate for 86 participants enrolled in this HF collaborative care study was 10.5%. In comparison, among 596 discharged patients with HF who received usual care in the same period, 23.5% were readmitted to the hospital.38
This study, although small, demonstrates principles promulgated in the 2021 ECDP.3 The HF team needs considerable and overlapping expertise in diagnosis, treatment (with an emphasis on titration and adherence), and patient education. They need to be knowledgeable about HF’s psychosocial impact, palliative care, and comorbid conditions. Beyond these primarily clinical skills, they need reliable inpatient and outpatient electronic medical records that communicate with each other; reliable, cost-effective electronic devices, wearable activity monitors, and secure mobile technologies.3
Investigational Approaches, Emerging Therapies
Cardiac Myosin Activator
Medications that increase cardiac contractility in HF have tended to have mechanism-related AE profiles. Omecamtiv mecarbil (OM) is a first-in-class cardiac myosin activator. It increases cardiac contractility by binding to the catalytic S1 domain of cardiac myosin. It has reduced heart rate, peripheral vascular resistance, mean left arterial pressure, and left ventricular end-diastolic pressure in animal models. Additionally, preclinical and clinical studies suggest OM may improve cardiac output and stroke volume, decrease ventricular wall stress, reverse ventricular remodeling, promote sympathetic withdrawal, and initiate systolic wall thickening. Three trials have evaluated OM’s tolerability, kinetics, and efficacy in the management of HF.39-42
The Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF).40 is a phase 3, international, multicenter, randomized, double-blind, placebo-controlled, event-driven CV outcomes study that enrolled 8256 patients. Eligible patients with chronic HFrEF symptoms aged 18 to 85 years were either hospitalized for HF, or had an urgent medical visit for HF (urgent clinic visit, emergency department visit, or hospitalization) within 12 months of screening. Patients were randomly assigned to receive OM twice daily or placebo. At a median of 21.8 months, CV death or HF event (the primary outcome) occurred in 37% of the OM-treated patients and in 39.1% of placebo-treated patients (HR, 0.92; 95% CI, 0.86-0.99; P = .03). OM did not produce a significant effect on CV death (HR, 1.01; 95% CI, 0.92-1.11), first hospitalization for HF (HR, 0.95; 95% CI, 0.87-1.03), or death from any cause (HR, 1.00; 95% CI, 0.92-1.09).40
Emerging Aldosterone Antagonists
Aldosterone antagonists have been associated with several treatment-limiting AEs. Aldosterone increases water retention and potassium reabsorption and induces proinflammatory activity, creating progressive fibrotic damage in the heart and kidney. As noted above, aldosterone receptor blockers can slow some components of HF progression. Hyperkalemia, however, is common. Investigators are working on a new generation of nonsteroidal mineralocorticoid receptor antagonists that might reduce the potential for hyperkalemia.43,44 Apararenone, esaxerenone, and finerenone are currently in clinical trials.43,44
Dual SGLT1/SGLT2 Inhibitor
The Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial recently evaluated sotagliflozin, an oral SGLT1/SGLT2 inhibitor, for use in patients with diabetes with recent worsening HF.45 Inhibiting SGLT2 diminishes renal glucose reabsorption, while inhibiting SGLT1 impairs glucose reabsorption within the gastrointestinal tract. SOLOIST-WHF was designed to determine if an SGLT1/SGLT2 inhibitor would reduce the risk of CV death, HF hospitalization, or urgent clinic visits for HF in patients with type 2 diabetes and recent worsening HFrEF or HFpEF when given shortly after an acute HF exacerbation. This multicenter, double-blind trial enrolled 1222 patients with diabetes who were recently hospitalized for worsening HF. Patients received sotagliflozin 200 mg daily (titrated to 400 mg daily if tolerated) or placebo and were followed for roughly 9 months. The primary end point occurred in 51% of patients on sotagliflozin and 76.3% of patients taking placebo (HR, 0.67; 95% CI, 0.52-0.85; P <.001). CV death was not statistically significantly different between arms (10.6% vs 12.5%; HR, 0.84; 95% CI, 0.58-1.22). The trial’s results were consistent across subgroups including patients with HFrEF or HFpEF. However, the trial closed early and was not powered to determine if these benefits would occur in a larger study population. Nonetheless, the SOLOIST-WHF trial has opened the door for further study of SGLT inhibitors in patients with HFpEF.45 However, the FDA refused to approve a New Drug Application for sotagliflozin on March 3, 2021, for the indication as an adjunct therapy in type 1 diabetes, which was submitted by Sanofi in partnership with Lexicon before Sanofi left the partnership. Therefore, sotagliflozin faces additional regulatory hurdles if it is to be eventually utilized in the management of HF.46
Conclusions
The changing demographics of patients hospitalized with HF and the disease’s increasing prevalence creates a sense of urgency to find new and better treatment approaches. Recent research and FDA approvals suggest that pharmacists and managed care administrators need to examine their protocols and prior authorization processes to ensure that the care they provide is in keeping with current evidence and guideline recommendations.
Author affiliations: Christopher Betz, PharmD, BCPS, FKSHP, FASHP, is a professor in the Department of Pharmacy Practice, Sullivan University College of Pharmacy and Health Sciences; clinical assistant professor of medicine in the Division of Cardiovascular Medicine, University of Louisville School of Medicine; and cardiology clinical pharmacy specialist, Jewish Hospital Rudd Heart & Lung Center—UofL Health, Louisville, KY.
Funding source: This activity is supported by an educational grant from Merck Sharp & Dohme Corp.
Author disclosure: Dr Betz has no relevant financial relationships with commercial interests to disclose.
Author information: Acquisition, analysis, and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and final approval of manuscript.
Address correspondence to: cbetz@sullivan.edu
Medical writing and editorial support provided by: Jeannette Y. Wick, RPh, MBA, FASCP
REFERENCES
1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi: 10.1161/CIR.0000000000000950
2. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee [published correction appears in J Am Coll Cardiol. 2020;75(1):132]. J Am Coll Cardiol. 2019;74(15):1966-2011. doi: 10.1016/j.jacc.2019.08.001
3. Writing Committee, Maddox TM, Januzzi JL Jr, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772-810. doi: 10.1016/j.jacc.2020.11.022
4. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240-327. doi: 10.1161/CIR.0b013e31829e8776
5. Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure [published online February 7, 2021]. J Card Fail. 2021;S1071-9164(21)00050-6. doi: 10.1016/j.cardfail.2021.01.022
6. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953-1959. doi: 10.1056/NEJMoa032566
7. Yoon S, Eom GH. Heart failure with preserved ejection fraction: present status and future directions. Exp Mol Med. 2019;51(12):1-9. doi: 10.1038/s12276-019-0323-2
8. Chang PP, Chambless LE, Shahar E, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113(3):504-510. doi: 10.1016/j.amjcard.2013.10.032
9. American Heart Association. Stages of heart failure and recommended therapy by stage. Accessed March 7, 2021. www.heart.org/-/media/files/health-topics/heart-failure/rahf-guidelines-toolkit-algorithm.pdf?la=en
10. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016-1022. doi: 10.1016/j.amjcard.2007.11.061
11. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474
12. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review [published correction appears in JAMA. 2020;324(20):2107]. JAMA. 2020;324(5):488-504. doi: 10.1001/jama.2020.10262
13. New York Heart Association (NYHA) Functional Classification. American Heart Association. Accessed March 7, 2021. www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure
14. Selvaraj S, Claggett B, Pozzi A, et al. Prognostic implications of congestion on physical examination among contemporary patients with heart failure and reduced ejection fraction: PARADIGM-HF. Circulation. 2019;140(17):1369-1379. doi: 10.1161/CIRCULATIONAHA.119.039920
15. Dickstein K, Vardas PE, Auricchio A, et al; Task Force on Acute Heart Failure of the European Society of Cardiology. 2010 focused update of ESC guidelines on device therapy in heart failure: an update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J. 2010;31(21):2677-2687. doi: 10.1093/eurheartj/ehq337
16. Ponikowski P, Voors AA, Anker SD, et al; Authors/Task Force Members. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128
17. Lindenfeld J, Albert NM, Boehmer JP, et al; Heart Failure Society of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1-194. doi: 10.1016/j.cardfail.2010.04.004
18. Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2014;2(6):663-670. doi: 10.1016/j.jchf.2014.09.001
19. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the omapatrilat cardiovascular treatment vs. enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103-111. doi: 10.1016/j.amjhyper.2003.09.014
20. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077
21. Entresto. Prescribing information. Novartis; 2021. Accessed April 8, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/207620s018lbl.pdf
22. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure [published correction appears in N Engl J Med. 2019;380(11):1090]. N Engl J Med. 2019;380(6):539-548. doi: 10.1056/NEJMoa1812851
23. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819-829. doi: 10.1016/S0140-6736(20)31824-9
24. McMurray JJ, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303
25. Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi: 10.1056/NEJMoa2022190
26. Jhund PS, Ponikowski P, Docherty KF, et al. Dapagliflozin and recurrent heart failure hospitalizations in heart failure with reduced ejection fraction: an analysis of DAPA-HF. Published online April 9, 2021. doi: 10.1161/CIRCULATIONAHA.121.053659
27. Invokana. Prescribing information. Janssen Pharmaceuticals, Inc; 2020. Accessed April 8, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/204042s036lbl.pdf
28. Farxiga. Prescribing information. AstraZeneca Pharmaceuticals LP; 2020. Accessed April 8, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf
29. Jardiance. Prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc; 2020. Accessed April 8, 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2020/204629s023lbl.pdf
30. Merck announces US FDA approval of Verquvo (vericiguat). News release. Merck. January 20, 2021. Accessed March 7, 2021. www.merck.com/news/merck-announces-u-s-fda-approval-of-verquvo-vericiguat/
31. Verquvo. Prescribing information. Merck Sharp & Dohme Corp; 2021. Accessed April 9, 2021. www.merck.com/product/usa/pi_circulars/v/verquvo/verquvo_pi.pdf
32. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883-1893. doi: 10.1056/NEJMoa1915928
33. Shim CY. Heart failure with preserved ejection fraction: the major unmet need in cardiology. Korean Circ J. 2020;50(12):1051-1061. doi: 10.4070/kcj.2020.0338
34. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi: 10.1056/NEJMoa1313731
35. Solomon SD, McMurray JJV, Anand IS, et al; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609-1620. doi: 10.1056/NEJMoa1908655
36. Solomon SD, Vaduganathan M, Claggett BL, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141(5):352-361. doi: 10.1161/CIRCULATIONAHA.119.044586
37. Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–33. doi: 10.1016/s0735-1097(03)00947-1
38. Boykin A, Wright D, Stevens L, Gardner L. Interprofessional care collaboration for patients with heart failure. Am J Health Syst Pharm. 2018;75(1):e45-e49. doi: 10.2146/ajhp160318
39. Teerlink JR, Diaz R, Felker GM, et al; GALACTIC-HF Investigators. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384(2):105-116. doi: 10.1056/NEJMoa2025797
40. Bavry AA. Global approach to lowering adverse cardiac outcomes through improving contractility in heart failure - GALACTIC-HF. Accessed March 7, 2021. www.acc.org/Latest-in-Cardiology/Clinical-Trials/2020/11/11/21/01/GALACTIC-HF
41. Teerlink JR, Felker GM, McMurray JJ, et al; COSMIC-HF Investigators. Chronic oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388(10062):2895-2903. doi: 10.1016/S0140-6736(16)32049-9
42. Patel PH, Nguyen M, Rodriguez R, Surani S, Udeani G. Omecamtiv mecarbil: a novel mechanistic and therapeutic approach to chronic heart failure management. Cureus. 2021;13(1):e12419. doi: 10.7759/cureus.12419
43. Capelli I, Gasperoni L, Ruggeri M, et al. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J Nephrol. 2020;33(1):37-48. doi: 10.1007/s40620-019-00600-7
44. Sueta D, Yamamoto E, Tsujita K. Mineralocorticoid receptor blockers: novel selective nonsteroidal mineralocorticoid receptor antagonists. Curr Hypertens Rep. 2020;22(3):21. doi: 10.1007/s11906-020-1023-y
45. Bhatt DL, Szarek M, Steg PG, et al; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384(2):117-128. doi: 10.1056/NEJMoa2030183
46. Department of Health and Human Services, Food and Drug Administration. Proposal to refuse to approve a new drug application for sotagliflozin oral tablets 200 milligrams and 400 milligrams; opportunity for a hearing. Federal Register 86 (40). 2021;12471-12473. March 3, 2021. Accessed May 5, 2021. www.federalregister.gov/documents/2021/03/03/2021-04342/proposal-to-refuse-to-approve-a-new-drug-application-for-sotagliflozin-oral-tablets-200-milligrams
