- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
FAQ: What to Know About the Vaccine Rollbacks and What They Mean Long-Term
With the CDC’s decision to roll back recommendations to vaccinate for several conditions, questions have arisen as to what that may mean in the US.
On January 5, 2026, the CDC announced that it would be reducing the childhood immunization schedule in the US from 17 to 11 vaccinations.1 Although these guidelines are not a mandate, this decision could have some notable implications for the future of childhood vaccines and may affect the prevention of diseases that affect children in large numbers. Questions remain for parents about what this means for their children in the long term. Below, we aim to answer some of the most pressing questions surrounding this decision.
Which vaccine recommendations were rolled back?
The CDC announced that recommendations for vaccines for respiratory syncytial virus, hepatitis A, hepatitis B, dengue, and 2 types of bacterial meningitis were rolled back.2 The vaccinations for flu, hepatitis A, hepatitis B, rotavirus, and bacterial meningitis are now recommended based on shared clinical decision-making.
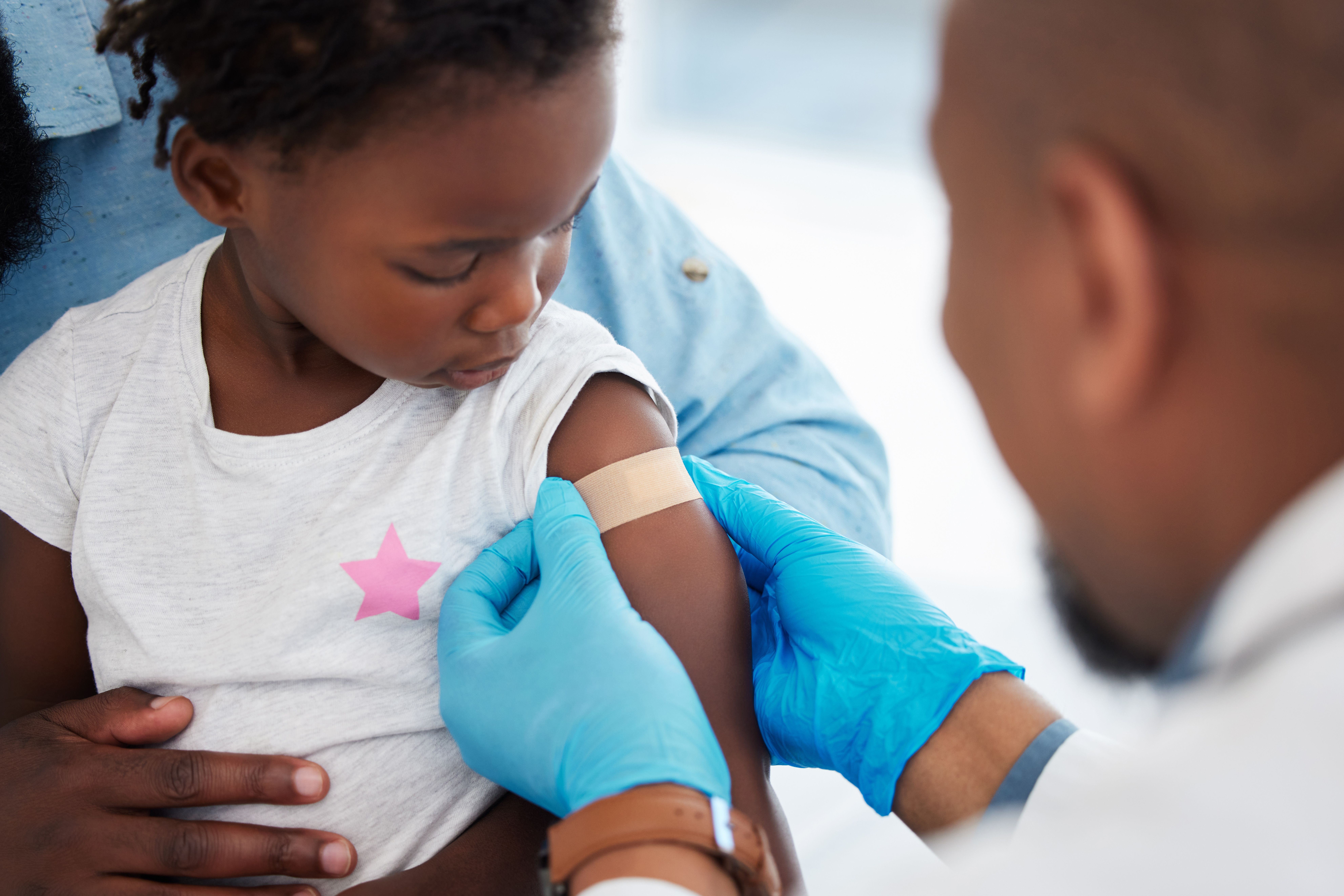
With the CDC rolling back vaccine recommendations, questions have arisen on what this would mean for children in the US | Image credit: Nina Lawrenson/peopleimages.com - stock.adobe.com
What does shared clinical decision-making mean with regard to the CDC Advisory Committee on Immunization Practices (ACIP) recommendations?
The ACIP-described shared clinical decision-making vaccinations are individually based and informed by a decision process between the health care provider and patient or parent/guardian. Providers will be unable to characterize these vaccinations as routine or catch-up vaccinations with parents, and these vaccines will not have risk-based recommendations.3 According to data collected after the COVID-19 vaccine was recommended based on shared clinical decision-making in 2025, Americans are often confused by what shared clinical decision-making entails.4 A total of 68% of those surveyed understood that shared decision-making is a discussion between the health care provider and the patient, or in the case of children, the parent or guardian. However, 22% believed that it meant that taking the vaccine would benefit some but not all patients, and 13% were not sure what shared decision-making meant in the context of a new vaccine. With these changes, parents may not get the vaccine for their child due to not understanding the wordings in the guideline.
Why were these changes made?
According to the CDC, these changes were made as a response to evaluating vaccine recommendations of other peer nations.5 The CDC concluded that the US was an outlier in the number of vaccines recommended when comparing with other developed nations. Before this update, the US vaccination schedule aligned with those of Canada, Britain, Germany, and Australia. The new CDC guidelines now mirror Denmark's 10-disease immunization schedule, though experts have questioned this comparison,6 noting that Denmark represents an outlier among wealthy nations. Key differences contributing to the previous vaccine recommendations include population size and health care structure. The United States has a population approximately 57 times larger than Denmark and incurs higher costs for severe pediatric illness, whereas Denmark maintains universal health care coverage.
Are these vaccines still safe and available?
All vaccines will remain available for all parents to approve for their child.7 According to the American Academy of Pediatrics, vaccines are “held to the highest scientific standards because they are given to healthy people.”8 Vaccines are tested on tens of thousands of study participants and compared against a placebo when no available vaccine exists. Vaccines are continuously monitored for safety even after approval by the FDA.
Will insurance still cover the vaccines?
AHIP has confirmed that all vaccines will continue to be covered, even if they are not universally recommended.9 Private insurers (including Aetna, UnitedHealthcare, and Blue Cross Blue Shield), Medicaid, the Children's Health Insurance Program, and the Vaccines for Children program will all continue covering these vaccines through 2026, including for uninsured children.
Do states have to follow these guidelines?
States are not required to adopt these guidelines. School vaccination requirements are individually determined by each state, though these CDC recommendations typically inform state-mandated vaccine policies.5 In the past, ACIP would review and update the CDC schedule each year before states turned to it to determine which vaccines to mandate for school attendance. It is possible that some states could adopt the new guidelines, whereas others could continue to use previous guidance.
References
- Grossi G. CDC reduces US childhood immunization schedule from 17 to 11 diseases. AJMC®. January 5, 2026. Accessed January 15, 2026. https://www.ajmc.com/view/cdc-reduces-us-childhood-immunization-schedule-from-17-to-11-diseases
- Lovelace B Jr, Edwards E, Fattah M, Bendix A. RFK Jr. overhauls childhood vaccine schedule to resemble Denmark’s in unprecedented move. NBC News. January 5, 2026. Accessed January 15, 2025. https://www.nbcnews.com/health/health-news/rfk-jr-vaccines-overhaul-kids-denmark-fewer-childhood-shots-rcna250055
- ACIP shared clinical decision-making recommendations. CDC. January 7, 2025. Accessed January 26, 2026. https://www.cdc.gov/acip/vaccine-recommendations/shared-clinical-decision-making.html
- Soucheray S. Confusion surrounds CDC’s ‘shared clinical decision-making’ paradigm for childhood vaccines. CIDRAP. January 6, 2026. Accessed January 15, 2026. https://www.cidrap.umn.edu/childhood-vaccines/confusion-surrounds-cdc-s-shared-clinical-decision-making-paradigm-childhood
- CDC acts on presidential memorandum to update childhood immunization schedule. News release. CDC. January 5, 2026. Accessed January 15, 2026. https://www.cdc.gov/media/releases/2026/2026-cdc-acts-on-presidential-memorandum-to-update-childhood-immunization-schedule.html
- Godoy M. Should the U.S. model its vaccine policy on Denmark’s? Experts say we’re nothing alike. NPR. December 26, 2025. Accessed January 26, 2026. https://www.npr.org/2025/12/26/nx-s1-5656214/childhood-vaccination-denmark-rfk-policy
- Bartels M. How CDC’s vaccine rollback will affect winter respiratory virus season. Scientific American. January 14, 2026. Accessed January 15, 2026. https://www.scientificamerican.com/article/rfk-jr-s-new-kids-vaccine-guidelines-will-worsen-flu-and-other-winter/
- Fact checked: childhood vaccines are carefully studied—including with placebos—to ensure they’re safe and effective. American Academy of Pediatrics. Updated May 12, 2025. Accessed January 15, 2026. https://www.aap.org/en/news-room/fact-checked/fact-checked-childhood-vaccines-are-carefully-studiedincluding-with-placebosto-ensure-theyre-safe-and-effective/
- Jacoby S. CDC no longer recommends 6 vaccines for all kids. Will insurance still cover them? Today. January 7, 2026. Accessed January 15, 2026. https://www.today.com/health/news/new-cdc-vaccine-schedule-insurance-coverage-rcna252456
