- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Emerging Therapies Shine Hope on Small Cell Lung Cancer Treatment
Newer treatments target pathways like Delta-like ligand 3, poly-ADP-ribose polymerase, and histone deacetylase to address small cell lung cancer's aggressive nature and resistance to conventional therapy.
Recent trial advancements have introduced several emerging therapies for SCLC. | Image credit: Dr_Microbe – stock.adobe.com
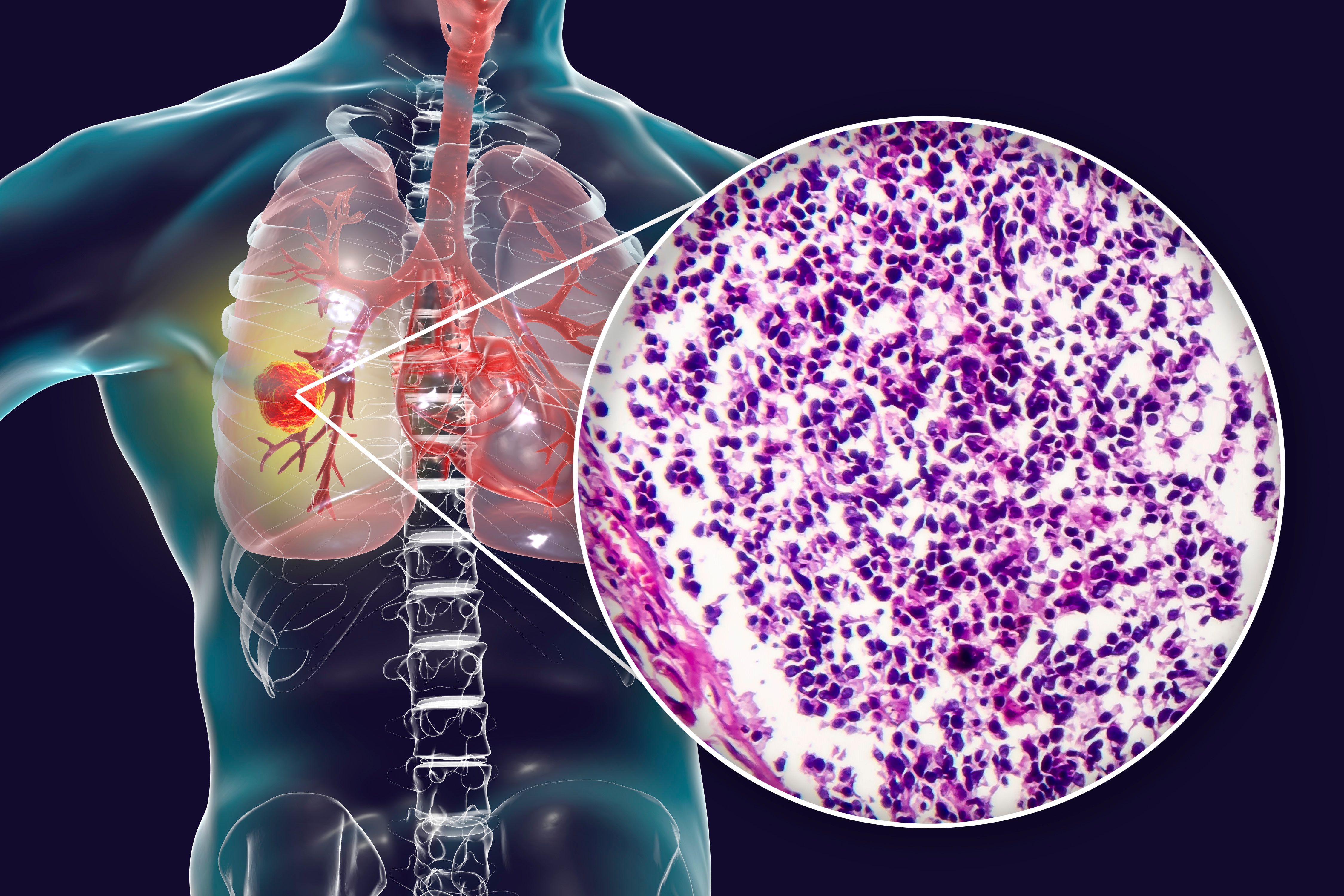
Small cell lung cancer (SCLC) represents about 15% of all lung cancer cases and remains a highly aggressive and difficult-to-treat malignancy. Standard first-line treatments like platinum-etoposide chemotherapy combined with immunotherapy have extended survival, but they offer only modest benefits, especially for relapsed SCLC.1
However, a growing pipeline of novel therapies targeting specific molecular pathways offers renewed hope, according to a recent review published in Cancers.
Emerging Therapies Target Critical Pathways
Recent trial advancements have introduced 10 emerging therapies mentioned in the review, including immune checkpoint inhibitors (ICIs), Delta-like ligand 3 (DLL3) inhibitors, and poly-ADP-ribose polymerase (PARP) inhibitors, which offer new options to combat resistance and improve survival outcomes.
PD-L1 inhibitors are already part of first-line treatment and continue to show incremental benefits. In clinical trials like KEYNOTE-604 (NCT03066778) and IMpower133 (NCT02763579), when added to standard chemotherapy, pembrolizumab extended the median survival from 10.5 to 12.9 months while atezolizumab extended it from 10.3 to 12.3 months, both compared with placebo.2,3 Although these therapies have reshaped the treatment landscape, there are still challenges in identifying biomarkers to better predict who will respond.1
According to the authors, one of the most promising developments is tarlatamab, a DLL3-targeting bispecific T-cell engager that redirects T cells to attack DLL3-expressing cancer cells. In the pivotal DeLLphi-301 trial (NCT05060016), a biweekly 10-mg dose of tarlatamab achieved an objective response rate of 35.3% and a median overall survival of 20.3 months.
“Tarlatamab, which received accelerated FDA approval in May 2024 as a promising treatment for patients with extensive-stage SCLC, offers hope for improved outcomes,” the authors noted. “However, its continued approval may depend on demonstrating clinical benefit in confirmatory trials, such as the ongoing DeLLphi-304 trial (NCT03319940), where the drug is expected to meet its primary end points.”
Other targeted therapies, including PARP inhibitors like olaparib and veliparib, aim to exploit SCLC’s DNA repair vulnerabilities. Although initial trials yielded limited improvements in survival, the authors suggest combining PARP inhibitors with other agents, such as immune checkpoint inhibitors or WEE1 inhibitors, for the chance of greater treatment efficacy. Findings from preclinical studies suggest these combinations could overcome resistance and lead to more durable responses.
Reducing Toxicities and Advancing Precision Medicine
A major hurdle in SCLC treatment is the systemic toxicity caused by chemotherapy and some newer therapies, but novel drug delivery systems like antibody-drug conjugates (ADCs) are helping to mitigate these effects, according to the review.
Sacituzumab govitecan—an ADC-targeting Trop-2—has shown tumor shrinkage in 60% of heavily pretreated patients, with a median duration of response of 5.7 months among the 50-person cohort. Similarly, epigenetic therapies like histone deacetylase (HDAC) inhibitors—including JBI-802, which has orphan drug designations for SCLC and acute myeloid leukemia—are being investigated for their ability to inhibit neuroendocrine tumor growth in both normal and MYC-amplified variants.
Precision medicine is also a growing focus, as researchers aim to integrate biomarkers and molecular profiling into clinical treatment planning. According to the authors, subtyping SCLC into categories like SCLC-A, SCLC-N, and others could allow clinicians to tailor therapies to individual patients based on tumor biology.
“Emerging treatments targeting novel pathways, like DLL3 and HDAC, show promise, while collaboration among researchers and industry leaders is crucial for translating innovations into clinical practice,” they said. “Ongoing research into resistance mechanisms and combination therapies offers hope for achieving durable responses and advancing the standard of care.”
References
1. Das S, Samaddar S. Recent advances in the clinical translation of small-cell lung cancer therapeutics. Cancers (Basel). 2025;17(2):255. doi:10.3390/cancers17020255
2. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369-2379. doi:10.1200/JCO.20.00793
3. Mansfield AS, Każarnowicz A, Karaseva N, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol. 2020;31(2):310-317. doi:10.1016/j.annonc.2019.10.021
