- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Disease Burden of Relapsing-Remitting Multiple Sclerosis
This publication was sponsored and written in partnership with Bristol-Myers Squibb.
EPIDEMIOLOGY
Multiple sclerosis (MS) is a central nervous system (CNS) disorder that involves inflammation, demyelination, and degenerative changes that eventually lead to disability if untreated.1 Approximately 300,000 to 400,000 individuals are thought to be affected by MS in the United States, but a 2017 estimate of prevalence based on observed increases in Department of Veterans Affairs and Intercontinental Marketing Services data sets, suggests that up to 913,925 individuals could be affected by MS in the United States.2
MS is often diagnosed in individuals between 20 and 40 years of age, with women accounting for approximately two-thirds of cases; however, onset can also occur in pediatric and older adult age groups.1,3 Although a higher rate of MS has been observed in White Americans than other racial groups (eg, Asian Americans and Hispanic Americans),4 recent studies suggest that Black women have a higher risk than previously reported and Black and Hispanic patients may have more rapid disease progression than White patients.1,4
DISEASE COURSE AND IMPORTANCE OF EARLY TREATMENT
Although the course of MS varies, 85% to 90% of patients present with a pattern of relapses and remissions of neurologic symptoms in the early stages of the disease, with clinical events that are usually associated with CNS inflammation.1 If untreated, this relapsing-remitting course is followed by a pattern of progressive worsening with few relapses or magnetic resonance imaging (MRI) activity, which is known as secondary progressive MS.1 Prompt treatment to minimize disease relapses in the early stages is essential because the mechanisms of CNS repair and remodeling that can partially compensate for the MS-induced damage in the early stages do eventually fail to keep up, leading to steady progression of physical and mental disability without remission (Figure 1).3 Relapses in the first few years of the disease have been associated with the future level of disability, and a short interval of time between the first and second relapse is a strong predictor of disability progression.3 In the relapsing-remitting course, MS is often characterized by actively demyelinating plaques associated with inflammation and disruption to the blood-brain barrier. These plaques (or lesions) tend to shrink over time in chronic MS, and this loss of lesion volume is a key contributor to brain atrophy.5
BURDEN OF DISABILITY
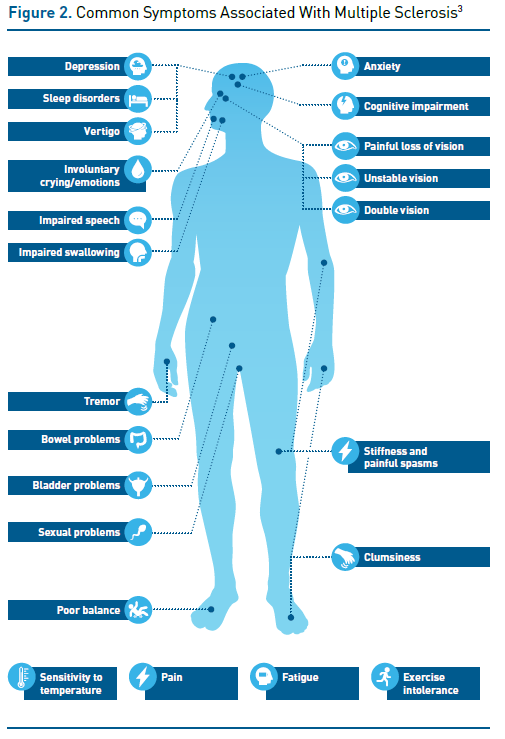
In relapsing-remitting MS, symptoms that appear in the form of relapse (also known as an attack or bout) may include sensory symptoms (eg, numbness, tingling, or burning pain), motor symptoms (eg, weakness, stiffness, clumsiness, difficulty walking, impairments in speech and swallowing), visual disturbances, physical or mental fatigue that interferes with daily activities, mood disorders (eg, depression and anxiety), genitourinary and bowel dysfunction, tremor, and stiffness, among others (Figure 2).3
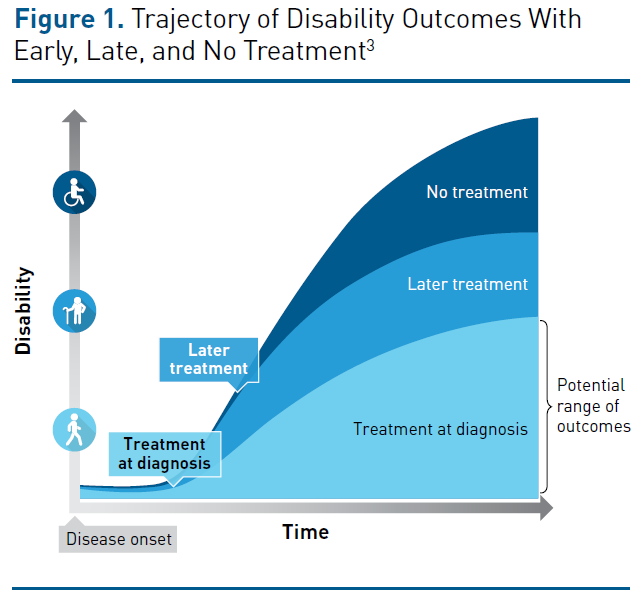
Symptoms associated with relapses often develop over a period of a few days, followed by a plateau; they subside over the next several weeks or months.3 During this time, neurological reserve and repair mechanisms enable remodeling and compensate for damage to the CNS. Although complete recovery often occurs in the early stages of MS, relapses may also result in sustained increases in disability, and incomplete recovery (due to exhaustion of neurological reserve) contributes to stepwise progression in disability. If relapsing-remitting disease transitions into secondary progressive MS, disability (particularly the ability to walk) worsens progressively independent of relapse activity.3 Without treatment, 50% to 60% of patients with relapsing-remitting MS develop secondary progressive disease within 15 to 20 years and, on average, lose the ability to walk unassisted for 100 meters after 14 years.3
Disability in patients with MS is typically assessed using the Expanded Disability Status Scale (EDSS), which assigns higher numeric values to greater levels of physical disability when assessing functional capacity in the pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral, and other systems.6 However, MS may negatively affect several other quality-of-life measures that are not adequately measured by EDSS, including cognition, vitality, mental health, and fatigue. Cognitive impairment in early-stage MS may reduce quality of life, negatively affect activities of daily life, and reduce the ability to work.3 Approximately 75% of patients with MS experience persistent or sporadic fatigue that often leads to lower quality of life.3
Rates of unemployment are also higher in patients with MS than in the general population, even among those with low levels of physical disability, suggesting that the cognitive impairment, fatigue, depression, and anxiety related to MS may negatively affect gainful employment.3 Furthermore, MS is associated with substantial indirect economic costs that may include loss of earnings by the patient’s unpaid caregiver, early withdrawal from an active lifestyle, and loss of productivity.7
UTILITY OF BIOMARKERS
Biomarkers for prognosis of MS may provide information about disease activity and whether conversion to another form of MS has occurred.8 Although some biomarkers have been shown to correlate with diagnosis and monitoring of disease activity, none have been shown to be specific for MS or to reliably predict an individual patient’s response to a therapy.8,9 For example, presence of oligoclonal bands in cerebrospinal fluid is predictive of conversion from clinically isolated syndrome (CIS) to MS and can be used to rule out other diagnoses and prognosticate conversion from CIS.9 White matter lesions on MRI indicate progression from CIS to clinically defined MS, although the relationship between T2-weighted white matter lesion load and clinical disability measured by EDSS varies among studies.9 There are some biomarkers of neurodegeneration that have been shown to correlate with disease severity and progression, but they do not predict relapses (which limits their clinical utility), because they generally fluctuate in correlation with disease activity that trails behind clinical relapses.9
MANAGEMENT OF MS
Comprehensive management of MS includes nonpharmacologic and pharmacologic therapies. Proper care contributes to the overall health of patients with MS and can impact disease progression and lifespan.3
Nonpharmacologic
To ensure the best outcomes in MS, it is important for patients to maintain a healthy diet, exercise, stop smoking, and lose weight (if indicated).3,10 Preventive care (such as immunizations) and the management of other medical conditions are also key.10 Patients with MS should also have access to programs that support their physical and emotional well-being.11 These include rehabilitation programs that promote functional mobility, safety, and independence for patients with MS (eg, speech and swallowing, memory and other cognitive functions). Mental health providers can lend support and education; they can also diagnose and treat depression, anxiety, and other conditions that are frequent in patients with MS.
Pharmacologic
Pharmacologic therapies for MS fall into 3 categories: those that treat relapses, those that target specific symptoms, and, most importantly, those that can modify the disease course (ie, disease-modifying therapies [DMTs]).12 Although mild relapses may not substantially impact daily activities or require treatment, severe attacks often necessitate a short course of intravenous or oral corticosteroids.13 Patients with MS can experience a multitude of symptoms and may require medications to manage them.
DMTs
Although no cure is available for MS, DMTs can reduce disease activity and slow the rate of progressive disability.3 Long-term studies show that early intervention with DMTs increases the likelihood of a favorable outcome compared with delayed intervention in patients with CIS or relapsing-remitting MS.3 DMTs have also been shown to increase time to second relapse and improve MRI outcomes, such as brain atrophy rate, in patients with CIS.3 Furthermore, DMTs have been shown to be more effective for slowing progression of disability in younger versus older patients and reducing the rate of relapse in younger (vs older) patients, patients with low (vs high) EDSS scores, and those with active lesions (vs no active lesions).3
GUIDELINE RECOMMENDATIONS FOR DMTS
Early, aggressive, and ongoing treatment with DMTs is important to reduce clinical and subclinical attacks and delay the onset of the progressive phase of disease, thereby helping to prevent onset of disability, prolong activity and engagement, and maintain quality of life.1 A 2018 report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology (AAN) recommended offering DMTs to patients with relapsing forms of MS who have had recent relapses and/or MRI activity, including those with 1 clinical episode who fulfill the 2010 International Criteria for MS, because of the demonstrated benefits with reducing relapses and MRI activity.14 Additionally, DMTs are associated with a delay in a second clinical relapse or new MRI-detected brain lesions in patients with a single demyelinating event and 2 or more MRI-detected brain or spinal cord lesions.14
Key goals of DMT for MS include reducing subclinical disease activity, minimizing loss of brain volume, and slowing disability progression. Early diagnosis enables initiation of DMT along with lifestyle interventions to preserve brain tissue and health and management of comorbidities such as blood pressure.3 According to AAN guidelines, choosing a DMT should involve shared decision-making between the patient and clinician, because considering patient preferences may help improve acceptance of and adherence to DMTs.14 Additionally, the AAN guidelines recommend that clinicians inform patients of the appropriate expectations of DMTs (ie, that they do not completely eliminate disease relapse and MRI activity) and possible need for additional treatment to address disease-related symptoms. After starting a DMT, clinicians should continue to monitor patients regularly for adherence, potential barriers to adherence, adverse events, tolerability, safety, and effectiveness.14 Reproductive plans should also be discussed and considered when selecting DMTs in patients of childbearing age.14
SUMMARY
MS typically progresses over time and can lead to irreversible disability if untreated; therefore, a comprehensive management plan that incorporates preventive care, healthy lifestyle choices, and prompt initiation of DMT is essential to slow progression of disease and optimize quality of life, physical function, and cognition. Ongoing follow-up and communication between the patient and provider are important to minimize disease activity and clinical symptoms and maximize patient quality of life.
REFERENCES
1. Costello K, Halper J, Kalb R, Skutnik L, Rapp R. Multiple Sclerosis Coalition. The use of disease modifying therapies in multiple sclerosis: principles and current evidence. National Multiple Sclerosis Society. Published July 2014. Updated September 2018. Accessed January 29, 2021. http://www.nationalmssociety. org/getmedia/5ca284d3-fc7c-4ba5-b005-ab537d495c3c/DMT_Consensus_MS_Coalition_color
2. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurol. 2019;92(10):e1029-e1040. doi:10.1212/ WNL.0000000000007035 Published correction appears in Neurology. 2019;93(15):688. doi:10.1212/ WNL.0000000000007915
3. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(suppl 1):S5-S48. doi:10.1016/j.msard.2016.07.003
4. Amezcua L, McCauley JL. Race and ethnicity on MS presentation and disease course. Mult Scler. 2020;26(5):561-567. doi:10.1177/1352458519887328
5. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183-193. doi:10.1016/S1474-4422(14)70256-X
6. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi:10.1212/wnl.33.11.1444
7. Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010;28(5):363-379. doi:10.2165/11532230-000000000-00000
8. Ziemssen T, Akgün K, Brück W. Molecular biomarkers in multiple sclerosis. J Neuroinflammation. 2019;16(272). doi:10.1186/s12974-019-1674-2
9. Housley WJ, Pitt D, Hafler DA. Biomarkers in multiple sclerosis. Clin Immunol. 2015;161(1):51-58. doi:10.1016/j.clim.2015.06.015
10. Diet, exercise & healthy behaviors. National Multiple Sclerosis Society. Accessed June 29, 2021. https:// www.nationalmssociety.org/Living-Well-With-MS/Diet-Exercise-Healthy-Behaviors
11. Comprehensive care. National Multiple Sclerosis Society. Accessed June 29, 2021. https://www.nationalmssociety.org/Treating-MS/Comprehensive-Care
12. Medications. National Multiple Sclerosis Society. Accessed June 29, 2021. https://www.nationalmssociety.org/Treating-MS/Medications
13. Managing relapses. National Multiple Sclerosis Society. Accessed June 29, 2021. https://www.nationalmssociety.org/Treating-MS/Managing-Relapses
14. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17);777- 788. doi:10.1212/WNL.0000000000005347 Published correction appears in Neurology. 2019;92(2):112. doi:10.1212/WNL.0000000000006722

