
- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Disease Burden of Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema
To claim CE credit for this activity, please visit
http://www.pharmacytimes.org/AMD-DME-suppl
ABSTRACT
Neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) are the leading causes of vision impairment in elderly patients and people living with diabetes, respectively. Common features of nAMD and DME include increased vascular permeability, inflammation, and neovascularization. Intravitreal administration of vascular endothelial growth factor (VEGF) inhibitors has been the gold standard for treating retinal diseases, and numerous studies have demonstrated their ability to stabilize disease progression and improve visual acuity. However, many patients struggle with the burden of frequent injections, experience a suboptimal treatment response, or lose vision over time. For these reasons, the outcomes of anti-VEGF treatment are often worse in the real-world compared with clinical trials.
Am J Manag Care. 2023;29(suppl 6):S75-S80. https://doi.org/10.37765/ajmc.2023.89387
Introduction
Neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) are leading causes of vision impairment among adults.1,2 The discovery of medications that inhibit vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2) has led to significant advances in the prevention of vision loss. The American Academy of Ophthalmology guidelines consider anti-VEGF therapy as first-line treatment for nAMD and DME, and each agent should be individually tailored based on discussions between the patient and physician.3,4 Despite these advances, the patient burden of disease remains significant, which has led to the pursuit of more effective treatment options in the management of nAMD and DME.
Epidemiology
There are nearly 20 million people in the United States who are living with some form of AMD; approximately 18 million people aged 40 years and older are living with early-onset AMD while an estimated 1.49 million have late-stage or vision-threatening nAMD.5 AMD is the leading cause of vision loss in people aged 60 years and older.6 The prevalence of AMD increases with age. Data from 2019 indicate the prevalence of AMD among adults aged 40 to 44 years was 2%, and it increased to 46% in people aged 85 years and older.5 Racial and ethnic groups are disproportionately affected by AMD. Caucasians and Asians are at greatest risk while non-Hispanic Blacks are least likely to develop AMD.7,8 There is also geographic variation in the prevalence of AMD. When broken down by state, the crude prevalence in the United States ranged from a low of 6.2% in the District of Columbia to a high of 18.3% in Florida.7 Cigarette smoking is widely considered the most important modifiable risk factor in AMD, and smoking cessation should be encouraged in patients who have AMD or are at risk for AMD.3 Data suggest that dietary intake may also have a prominent role in AMD. For example, adherence to a Mediterranean diet and increased consumption of foods high in carotenoids and omega-3 fatty acids has been beneficial in terms of reducing disease progression and risk of AMD.9
Compared with AMD, there are approximately 21 million people worldwide living with DME.10 Patients diagnosed with diabetes before age 30 years have a 10-year incidence rate for DME of approximately 20%; this rate approaches nearly 40% in patients diagnosed with diabetes after age 30 years.11 It is more likely that DME will occur in individuals with type 1 diabetes (T1D) than those with type 2 diabetes (T2D), although nearly 80% of individuals with T2D will develop some form of diabetic eye disease after 10 years of their initial diagnosis.11,12 Despite these data, an estimated 40% of people with diabetes skip recommended annual retinal screenings for diabetic eye disease. People with T2D should have an ophthalmic evaluation at initial diagnosis and at least annually thereafter, whereas people with T1D should have annual ophthalmic evaluation starting 5 years after the onset of disease.4
Pathophysiology of nAMD and DME
AMD is an eye disease that results from accumulation of lipids and subsequent degenerative changes to the macula, the part of the eye that provides sharp, central vision required for normal daily activities, such as reading and driving.13 The 2 fundamental forms of AMD are dry and wet. The dry form of AMD occurs without subsequent subretinal neovascularization and may culminate in geographic macular atrophy. Anti-VEGF therapy is not indicated for dry AMD. In contrast, wet AMD involves subretinal neovascularization and is treated by use of anti-VEGF agents. As wet AMD progresses from early- to late-stage disease, patients often experience rapid, progressive vision loss. This pathological process is referred to as choroidal neovascularization and occurs when abnormal blood vessels grow uncontrolled under the macula, which results in swelling, bleeding, and fibrosis.13
DME can be present alone or with any stage of diabetic retinopathy. It is a vision-threatening condition that occurs when blood vessels in the retina leak fluid into the macula.14 Several biological pathways have been implicated; however, the angiogenic, inflammatory, and oxidative stress pathways are most recognized. Increased levels of blood glucose promote inflammation, oxidative stress, and the development of intra- and extracellular advanced glycation end products, which activate the protein kinase C pathway. Protein kinase C mediates changes in retinal vascular permeability, blood flow, and the cellular response to angiogenic growth factors (eg, VEGF). Oxidative stress from free radical formation, overexpression of angiogenic growth factors, and increased production of protein kinase C all contribute to the vascular damage and subsequent macular edema characteristic of DME.14
Ang-Tie Signaling Pathway
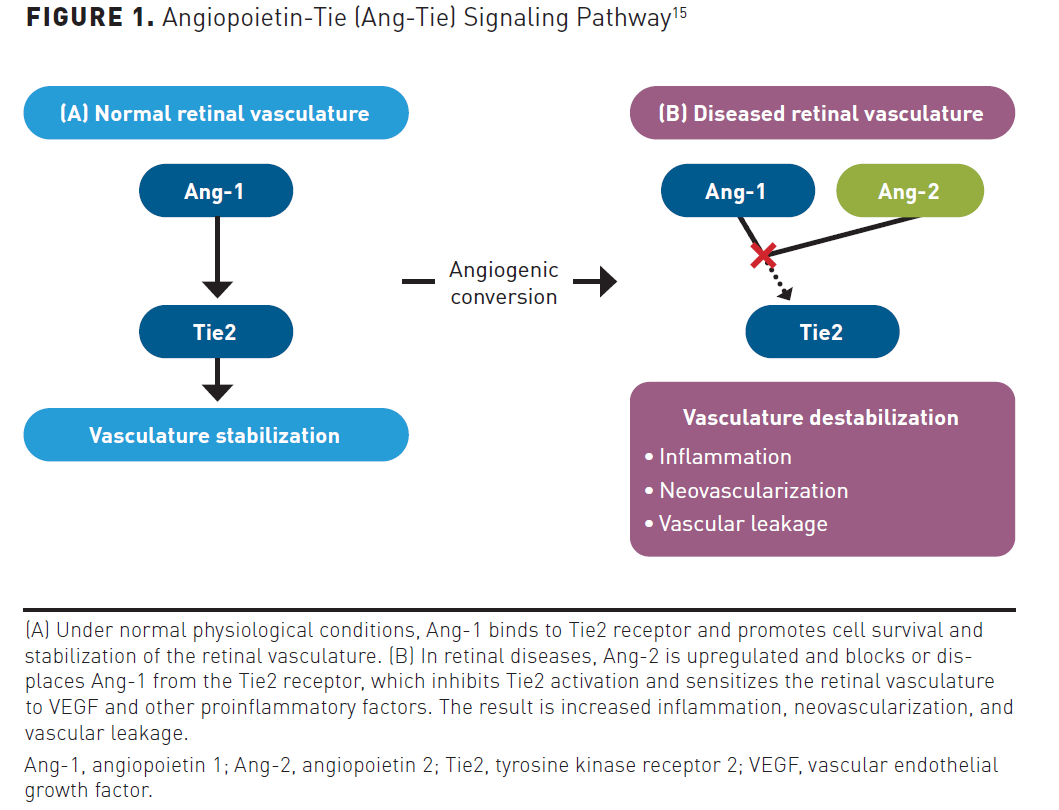
Increased vascular permeability, inflammation, and neovascularization are similar shared pathological processes that contribute to vision impairment in both nAMD and DME. Although the VEGF family has proven to be critical regulators of angiogenesis and vascular permeability in retinal diseases, the angiopoietin-tyrosine kinase with immunoglobulin and epidermal growth factor homology domains (Ang-Tie) pathway plays a significant role as well. The Ang-Tie pathway consists of 2 tyrosine kinase receptors (Tie1 and Tie2) and 4 ligands (Ang-1, Ang-2, Ang-3, and Ang-4). Ang-1 and Ang-2 have been studied extensively, whereas Ang-3 and Ang-4 are not as well characterized.15 Ang-1 is a Tie2 receptor agonist and is expressed by smooth muscle cells, pericytes, and mesenchymal cells. Under normal physiological conditions, Ang-1 binds to the Tie2 receptor and promotes stability of the retinal vasculature. Ang-2 is stored in Weibel-Palade bodies and is produced by endothelial cells. In retinal diseases, Ang-2 is overexpressed and has a negative effect on retinal vasculature, which includes pericyte detachment, suppression of the Tie2 receptor, and increased sensitization of blood vessels to VEGF-A and other proinflammatory mediators. Overall, the Ang-2–Tie2 signaling axis promotes inflammation, neovascularization, and vascular leakage (Figure 1).15 An increased understanding of the Ang-Tie signaling pathway has led to the recent FDA approval of faricimab-svoa, an immunoglobulin antibody that inhibits both VEGF-A and Ang-2 pathways and helps stabilize the retinal vasculature.16
Current Treatment Limitations and Unmet Needs
Intravitreal VEGF inhibitors, such as aflibercept, bevacizumab, and ranibizumab, are widely considered the gold standard for the treatment of nAMD and DME. Each of these treatments addresses subretinal choroidal neovascularization by inhibiting the physiologic effects of VEGF. When used as recommended, they effectively reduce risk of vision loss associated with nAMD and DME. Unfortunately, there are limitations that exist with anti-VEGF treatments, and they begin with the number of injections required to lessen disease progression.17,18
Treatment and monitoring is currently guided by zero tolerance for fluid, meaning the macula must be persistently fluid-free. Fluid accumulation in any amount indicates disease activity, which signals the retina specialist to increase the intensity of anti-VEGF therapy combined with more frequent monitoring.19 Therefore, despite its effectiveness, anti-VEGF treatment usually requires repeated intravitreal injections and more follow-up visits. Studies indicate that treatment-naïve eyes beginning anti-VEGF therapy for nAMD receive a median of 18 injections from a median of 21 clinic visits over the first 3 years.20 The need for repeat treatments is a significant burden on patients, families, and the health care system. Unfortunately, nonadherence rates ranging from 32% to 95% have been reported, and this continues to be one of the major challenges in preventing vision loss associated with retinal diseases.21
Safety
Intravitreal anti-VEGF treatments have several eye-related safety concerns, including blurred vision, conjunctival hemorrhage, eye pain, vitreous detachment, and vitreous floaters.17,18,22 Severe ocular adverse effects (AEs) that have been linked to anti-VEGF treatment include infectious and noninfectious endophthalmitis, retinal detachment, and increased intraocular pressure. Multiple systematic reviews indicate that serious AE rates are relatively similar among anti-VEGF treatments.23,24 However, while AE rates were similar between aflibercept and brolucizumab in clinical trials, the American Society of Retina Specialists released a statement in 2020 regarding several reported cases of vasculitis linked to brolucizumab, of which more than one-half were designated as occlusive retinal vasculitis.25 Accordingly, it is important that retina specialists monitor appropriately for this safety signal when treating patients with brolucizumab.
Systemic AEs associated with anti-VEGF treatments have been the subject of much debate. For example, a study indicated that mortality in patients who received bevacizumab within 3 months after stroke or transient ischemic attack was significantly different than in patients who were not exposed to bevacizumab (OR, 6.92; 95% CI, 1.88-25.43; P <.01).26 However, multiple follow-up studies have shown that anti-VEGF treatments do not increase the risk of systemic AEs, such as arterial thromboembolism, extraocular hemorrhage, stroke, or myocardial infarction.27,28 Study investigators indicated that caution might be advisable in patients with AMD who are at increased risk of hemorrhagic events when receiving ranibizumab.27
Understanding the Patient Experience
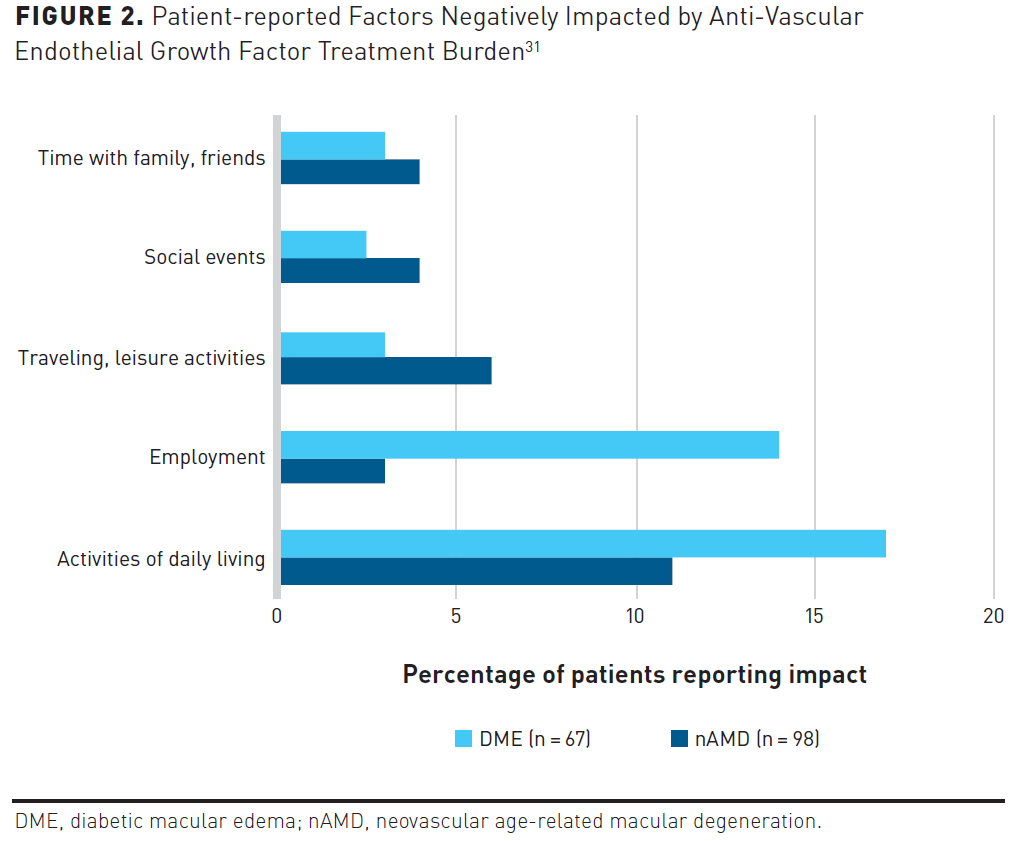
Patients have expressed myriad concerns about the treatment-related demands of anti-VEGF therapy as well as the burden imposed on caregivers, specifically the disruption to their work and social schedules.29,30 Patients report the average clinic visit can last up to 12 hours when factoring in travel and post-appointment recovery; however, it has been reported that as many as 21% of patients require more than 1 day to recover.31,32 In addition, regular optical coherence tomography monitoring is a critical component in anti-VEGF regimens that extends the dosing interval to detect intraretinal or subretinal fluid, which increases both cost and patient time in clinic.33 To determine the patient and caregiver experience with anti-VEGF treatment, researchers invited adult patients living with nAMD or DME who were treated with anti-VEGF therapy for at least 12 months to participate in a cross-sectional survey. Patient perspectives related to the burden of anti-VEGF treatment are described in Figure 2.31
Additionally, patient loss to follow-up is high. In a study of 2595 patients with DME who were treated with anti-VEGF therapy, 25.3% of patients failed to return for scheduled treatment and were considered lost to follow-up. Minority groups and those with lower incomes were most likely to not return.34 The effects of treatment discontinuation or interruption are detrimental to visual outcomes of patients with retinal disease. Some experts consider perfect compliance to be nearly impossible because many patients with nAMD or DME have significant comorbidities and must prioritize their health care needs.30 For instance, older patients and those with visual impairment may need to rely on others for transportation, which creates a significant barrier to optimal treatment adherence. Younger patients may find it difficult to take time away from work, or they may experience gaps in treatment due to unanticipated loss of employment.30 It is important for physicians to keep these issues in mind when deciding on therapeutic options for each patient. No single metric can predict which patients will return to clinic visits on time or who may be lost to follow-up.
Divergent Clinical Trials’ Efficacy and Real-World Effectiveness
The inability to reproduce results from randomized controlled trials in everyday practice is not uncommon.35 Numerous observational studies have found that patients with visual impairment are monitored and treated less frequently in real-world clinical practice compared with controlled settings. As a result, patients may experience worse vision outcomes.36-38 In addition, outcomes from clinical trials indicate that anti-VEGF treatments are sometimes less robust than is ideal. While anti-VEGF therapies generally stabilize or improve visual acuity (VA), subretinal fibrosis can occur as a natural course of AMD—despite treatment with anti-VEGF injections—and can lead to loss of vision. For instance, patients who had no ophthalmic scarring at enrollment in the Comparison of Age-related Macular Degeneration Treatment Trials (CATT) were administered 1 of 3 anti-VEGF treatment regimens and were followed for 2 years. At study conclusion, 480 of 1059 eyes developed macular scarring or fibrosis, which limited further improvements in VA despite continued treatment. Eyes with classic neovascularization, a thicker retina, and more fluid under the foveal center of the retina were more likely to develop fibrotic or nonfibrotic scarring.39
Long-term treatment involving repeated intravitreal injections raises another concern. For example, disease activity in nAMD and DME may continue or worsen if tachyphylaxis or tolerance develops. Tachyphylaxis is defined as a decrease in therapeutic drug response following repeated administrations; when the medication is interrupted for a short period of time, the clinical efficacy of the medication can usually be regained, but no extra benefit is achieved by increasing the dose.40 Tolerance is a progressive loss of drug efficacy with long-term use; an added clinical benefit can often be achieved by increasing the dose or shortening the dosing interval, but suspending treatment temporarily does not improve overall efficacy.40 Although a precise incidence of tachyphylaxis and tolerance related to anti-VEGF treatment is unavailable, some authors indicate it may be between 2% and 10%.41-43 When patients with nAMD lose drug response or experience treatment resistance to a particular anti-VEGF therapy, switching to another anti-VEGF treatment is a reasonable option to consider. For example, a meta-analysis that included 28 studies showed significant improvements in mean central retinal thickness after patients were switched from either ranibizumab or bevacizumab to aflibercept, with reductions of –61.90 μm (95% CI, –77.10 to –46.80; P <.001) at 6 months and –50.00 μm (95% CI, –63.20 to –36.80; P <.001) at 1 year.44
Ideally, patients express desire to maintain or restore their vision to a level that permits maximum independence. For many people, this translates to the ability to drive. However, studies indicate that only one-half of patients treated with anti-VEGF therapy for 24 months achieved the typical level of VA required for an unrestricted driver’s license.45 Another study evaluated binocular vision in 136 patients who had lost their driving privileges due to declining vision. At 1, 2, 3, and 4 years, just 29%, 36%, 39%, and 41% of patients, respectively, had regained adequate vision to be eligible for relicensure. Although these numbers are encouraging, between 60% and 70% of patients did not regain enough vision to drive and maintain their independence.46
Financial Constraints
The financial burden of retinal diseases can be a significant challenge for all patients. Large insurance deductibles, co-pays, and transportation-related concerns are noted barriers to strict adherence with anti-VEGF injection schedules.30 Many patients with nAMD and DME are Medicare beneficiaries, and Part B covers 80% of physician-administered drugs. Patients must cover the remaining 20% of the allowable reimbursement for both the drug and physician services associated with administering the drug.47 Out-of-pocket costs can accumulate rapidly, especially if indirect costs like transportation, work absenteeism, and loss of employment are considered.47 The mean annual expenses for visual impairment per patient in terms of US purchasing power parities range from $12,175 to $14,029 for moderate visual impairment; $13,154 to $16,321 for severe visual impairment; and $14,882 to $24,180 for blindness.48
Unaddressed Issues in a Multifactorial Disease
Though anti-VEGF treatments have changed the prognosis for many patients with nAMD and DME, researchers suggest that the lack of long-term restoration of VA and the subset of patients in whom treatment fails to elicit a response indicates the need to explore VEGF-independent mechanisms. Current therapies for nAMD and DME rely almost exclusively on inhibition of VEGF-A and Ang-2, though other pharmacologic targets are thought to contribute to retinal pathology.14,15 For example, patients with nAMD who were treated with bevacizumab have shown increased aqueous levels of several angiogenic biomarkers, including VEGF-C, hepatocyte growth factor, follistatin, and interleukin 8.49 Clinical studies have suggested a role for each of these vasogenic biomarkers in choroidal and retinal angiogenesis, which may represent new therapeutic targets in the pathophysiology of nAMD and DME.49 These biomarkers should be interpreted with caution as they may simply be a result of compensatory inflammatory and growth factor pathways engaged through VEGF inhibition.
Conclusions
As the treatment armamentarium for nAMD and DME expands, clinicians and policy makers need to be mindful of the treatment-related burdens for patients, caregivers, and the health care system. Numerous experimental agents and drug delivery systems are in development that may reduce the need for frequent intravitreal injections. In the same way that the anti-VEGF treatments were considered dramatic improvements when they were first approved, new medications and delivery systems may change the way that clinicians treat retinal disease.
Author affiliation: Arghavan Almony, MD, is senior retina partner and vice president, Carolina Eye Associates, PA, in Southern Pines, NC; and an adjunct assistant professor at Campbell University School of Medicine, Lillington, NC.
Funding source: This activity is supported by an educational grant from Genentech, a member of the Roche Group.
Author disclosure: Dr Almony has the following relevant financial relationship with a commercial interest to disclose: Consultant: Cardinal Health.
Authorship information: Critical revision of the manuscript for important intellectual content; supervision; and administrative, technical, or logistic support.
Address correspondence to: almony@gmail.com
Medical writing and editorial support provided by: Jeannette Y. Wick, RPh, MBA, FASB
REFERENCES
- Blindness and vision impairment. World Health Organization. Published October 13, 2022. Accessed February 16, 2023. www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment
- Diabetes: vision loss. Centers for Disease Control and Prevention (CDC). Updated December 19, 2022. Accessed January 23, 2023. www.cdc.gov/diabetes/managing/diabetes-vision-loss.html
- Age-related macular degeneration preferred practice pattern, 2019. American Academy of Ophthalmology (AAO). Published 2019. Accessed February 22, 2023. www.aao.org/education/preferred-practice-pattern/age-related-macular-degeneration-ppp
- Diabetic retinopathy preferred practice pattern, 2019. AAO. Published September 7, 2019. Accessed February 22, 2023. www.aaojournal.org/article/S0161-6420(19)32092-5/pdf
- Rein DB, Wittenborn JS, Burke-Conte Z, et al. Prevalence of age-related macular degeneration in the US in 2019. JAMA Ophthalmol. 2022;140(12):1202-1208. doi:10.1001/jamaophthalmol.2022.4401
- Age-related macular degeneration. Bright Focus Foundation. Updated December 8, 2022. Accessed February 19, 2023. www.brightfocus.org/macular/article/age-related-macular-facts-figures
- Prevalence of age-related macular degeneration (AMD). CDC. Updated October 31, 2022. Accessed January 22, 2022. www.cdc.gov/visionhealth/vehss/estimates/amd-prevalence.html
- Mahr MA, Hodge DO, Erie JC. Racial differences in age-related macular degeneration and associated anti-vascular endothelial growth factor intravitreal injections among Medicare beneficiaries. Ophthalmol Retina. 2018;2(12):1188-1195. doi:10.1016/j.oret.2018.05.005
- Chapman NA, Jacobs RJ, Braakhuis AJ. Role of diet and food intake in age-related macular degeneration: a systematic review. Clin Exp Ophthalmol. 2019;47(1):106-127. doi:10.1111/ceo.13343
- Yau JW, Rogers SL, Kawasaki R, et al; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. doi:10.2337/dc11-1909
- Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1-32. doi:10.1016/j.survophthal.2008.10.001
- Li JQ, Welchowski T, Schmid M, et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(1):11-23. doi:10.1007/s10654-019-00560-z
- Deng Y, Qiao L, Du M, et al. Age-related macular degeneration: epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021;9(1):62-79. doi:10.1016/j.gendis.2021.02.009
- Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010;88(3):279-291. doi:10.1111/j.1755-3768.2008.01501.x
- Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond). 2021;35(5):1305-1316. doi:10.1038/s41433-020-01377-x
- Vabysmo injection, for intravitreal use. Prescribing information. Genentech; January 2023. Accessed March 20, 2023. www.gene.com/download/pdf/vabysmo_prescribing.pdf
- Eylea injection, for intravitreal use. Prescribing information. Regeneron Pharmaceuticals, Inc.; February 2023. Accessed March 20, 2023. www.regeneron.com/downloads/eylea_fpi.pdf
- Lucentis for intravitreal injection. Prescribing information. Genentech; March 2018. Accessed March 20, 2023. www.gene.com/download/pdf/lucentis_prescribing.pdf
- Balaskas K, Amoaku WM, Cudrnak T, et al. Importance of anatomical efficacy for disease control in neovascular AMD: an expert opinion. Ophthalmol Ther. 2021;10(2):231-243. doi:10.1007/s40123-021-00342-5
- Bhandari S, Nguyen V, Arnold J, et al. Treatment outcomes of ranibizumab versus aflibercept for neovascular age-related macular degeneration: data from the Fight Retinal Blindness! Registry. Ophthalmology. 2020;127(3):369-376. doi:10.1016/j.ophtha.2019.10.006
- Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234-247. doi:10.1016/j.ophtha.2020.07.060
- Beovu injection. Prescribing information. Novartis Pharmaceuticals Corporation; December 2022. Accessed March 20, 2023. www.novartis.com/us-en/sites/novartis_us/files/beovu.pdf
- Moja L, Lucenteforte E, Kwag KH, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;9(9):CD011230. doi:10.1002/14651858.CD011230.pub2
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3(3):CD005139. doi:10.1002/14651858.CD005139.pub4
- Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050-1059. doi:10.1016/j.ophtha.2020.11.011
- Hanhart J, Comaneshter DS, Vinker S. Mortality after a cerebrovascular event in age-related macular degeneration patients treated with bevacizumab ocular injections. Acta Ophthalmol. 2018;96(6):e732-e739. doi:10.1111/aos.13731
- Thulliez M, Angoulvant D, Pisella PJ, Bejan-Angoulvant T. Overview of systematic reviews and meta-analyses on systemic adverse events associated with intravitreal anti-vascular endothelial growth factor medication use. JAMA Ophthalmol. 2018;136(5):557-566. doi:10.1001/jamaophthalmol.2018.0002
- Dalvin LA, Starr MR, Abou-Chehade JE, et al. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophthalmol. 2019;137(5):483-490. doi:10.1001/jamaophthalmol.2018.6891
- Ghanchi F, Bourne R, Downes SM, et al. An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis and glaucoma. Eye (Lond). 2022;36(6):1154-1167. doi:10.1038/s41433-021-01766-w
- Salabati M, Russ Soares R, Hsu J. Loss to follow-up after anti-VEGF for age-related macular degeneration and diabetic retinopathy; improving patient adherence and understanding of risk factors can preserve vision. Ret Physician. Published June 1, 2021. Accessed January 22, 2023. www.retinalphysician.com/issues/2021/june-2021/loss-to-follow-up-after-anti-vegf-for-age-related
- Holekamp NM, Veeral SS, Murtaza AK, et al. Patient and caregiver experience with anti-VEGF intravitreal injections to treat neovascular age-related macular degeneration and diabetic macular edema in the USA. Poster presented at: American Society of Retina Specialists (ASRS) 40th Annual Scientific Meeting; July 13-16, 2022; New York, NY. Accessed March 26, 2023. medically.gene.com/content/dam/pdmahub/restricted/ophthalmology/asrs-2022/ASRS-2022-poster-holekamp-patient-and-caregiver-experience-with-anti-vegf-intravitreal-injections.pdf
- Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725-31.e1. doi:10.1016/j.ajo.2015.06.023
- Holekamp NM. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25:S172-S181.
- Gao X, Obeid A, Aderman CM, et al. Loss to follow-up after intravitreal anti-vascular endothelial growth factor injections in patients with diabetic macular edema. Ophthalmol Retina. 2019;3(3):230-236. doi:10.1016/j.oret.2018.11.002
- Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 Ladder clinical trial. Ophthalmology. 2019;126(8):1141-1154. doi:10.1016/j.ophtha.2019.03.036
- Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220-226. doi:10.1136/bjophthalmol-2014-305327
- Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92,976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092-1101. doi:10.1016/j.ophtha.2013.11.031
- Ozturk M, Harris ML, Nguyen V, Barthelmes D, Gillies MC, Mehta H. Real-world visual outcomes in patients with neovascular age-related macular degeneration receiving aflibercept at fixed intervals as per UK licence. Clin Exp Ophthalmol. 2018;46(4):407-411. doi:10.1111/ceo.13085
- Daniel E, Toth CA, Grunwald JE, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656-666. doi:10.1016/j.ophtha.2013.10.019
- Wang Z, Li M, Yao Y, et al. Short-term results of switch from conbercept to bevacizumab or ranibizumab in eyes with persistent neovascular age-related macular degeneration. J Ophthalmol. 2020;2020:9340356. doi:10.1155/2020/9340356
- Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina. 2009;29(6):723-731. doi:10.1097/IAE.0b013e3181a2c1c3
- Eghøj MS, Sørensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2012;96(1):21-23. doi:10.1136/bjo.2011.203893
- Hara C, Wakabayashi T, Fukushima Y, et al. Tachyphylaxis during treatment of exudative age-related macular degeneration with aflibercept. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2559-2569. doi:10.1007/s00417-019-04456-2
- Spooner K, Hong T, Wijeyakumar W, Chang AA. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017;11:161-177. doi:10.2147/OPTH.S125676
- Bressler NM, Varma R, Mitchell P, et al. Effect of ranibizumab on the decision to drive and vision function relevant to driving in patients with diabetic macular edema: report from RESTORE, RIDE, and RISE trials. JAMA Ophthalmol. 2016;134(2):160-166. doi:10.1001/jamaophthalmol.2015.4636
- Ferløv Baselius NJ, Brynskov T, Falk MK, Sørensen TL, Subhi Y. Driving vision in patients with neovascular AMD in anti-VEGF treatment. Acta Ophthalmol. 2021;99(8):e1360-e1365. doi:10.1111/aos.14831
- Shah AR, Williams GA. Regulatory and economic considerations of retinal drugs. Dev Ophthalmol. 2016;55:376-380. doi:10.1159/000438965
- Köberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3(11):e003471. doi:10.1136/bmjopen-2013-003471
- Cabral T, Lima LH, Mello LGM, et al. Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmol Retina. 2018;2(1):31-37. doi:10.1016/j.oret.2017.04.004
2 Commerce Drive
Cranbury, NJ 08512
AJMC®
All rights reserved.