- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Defining a Path Toward Improved Heart Failure Care
ABSTRACT
Defining a path toward improved heart failure (HF) care is essential, as there is a clear need to improve HF treatment quality, outcomes, and value. This article reviews potential strategies to help improve the quality of HF clinical care and decrease costs.
To start, HF phenotyping may be useful in guiding patient treatment, as some phenotypes are associated with higher hospitalization costs and longer length of stay. Identifying and addressing social determinants of health that may be barriers to optimal health may improve management of HF and help to prevent disease progression. In addition, patient-reported outcomes can be useful for evaluating the effectiveness of treatment regimens and assessing which treatments lead to a genuine improvement in quality of life (QOL).
Recent innovations in payment reform have seen the implementation of value-based payment (VBP) models over the traditional fee-for-service (FFS) models. FFS models can lead to low-quality care focused on treating illness instead of supporting wellness initiatives. By contrast, VBP models aim to decrease excessive health care costs, thereby increasing incentives to hospitals that deliver high-quality patient care.
Further, novel care delivery approaches, such as hospital-at-home and other digital tools, can provide patients with lower-cost care and are associated with improved QOL, including reductions in hospital readmission.
Am J Manag Care. 2023;29:S195-S200. https://doi.org/10.37765/ajmc.2023.89418
For author information and disclosures, see end of text.
Introduction
Heart failure (HF) affects more than 6.5 million people in the United States alone, with approximately 550,000 new cases diagnosed annually.1 Overall, spending for HF comprises 1% to 2% of the US total health care expenditure,2 with hospitalizations accounting for a majority of the direct costs.2,3 In 2011, 3.3 million hospital readmissions occurred at an associated cost of $41.3 billion, with the overall cost of HF projected to rise to $69.8 billion by 2030.4,5
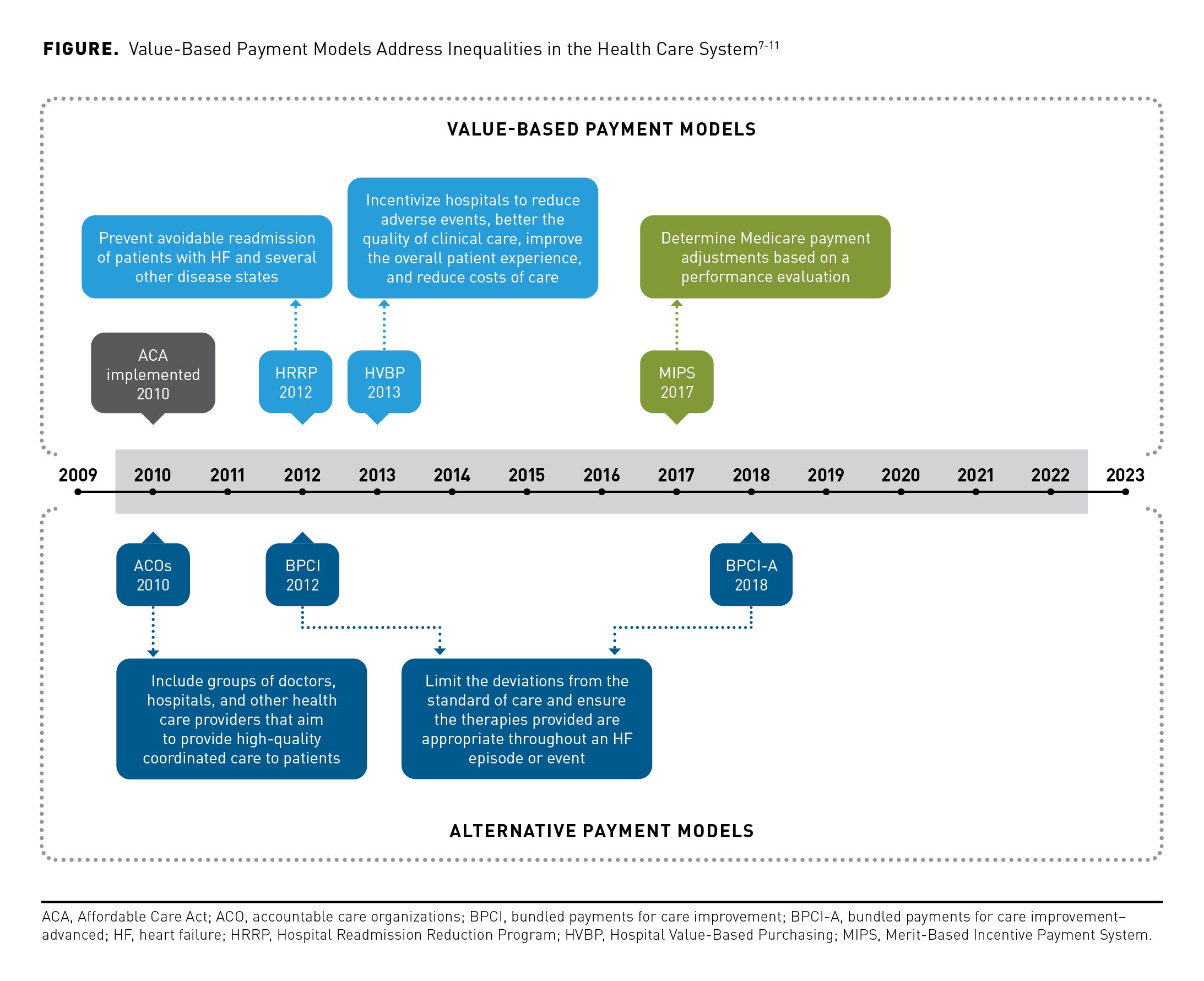
Given the economic burden of HF, health care systems are striving to implement value-based payment (VBP) models instead of traditional fee-for-service (FFS) models. While FFS models involve volume-based payments, VBP models offer incentives to physicians and institutions for meeting certain care quality standards.6 In this way, VBP models aim to address the fragmented, low-quality health care associated with FFS models (Figure).7-11 Thus, the goal of payment reform is to drive improvements in health care quality, positively influence treatment outcomes, and reduce costs for the HF patient population. To improve the quality of HF care and reduce costs, a path for improvement is needed that can incorporate additional patient data and metrics to define outcomes while implementing innovative new payment and care delivery models.
Improving Phenotyping by Incorporating New Measures
Traditionally, HF treatment has been based largely on determination of the left ventricular ejection fraction (LVEF).12 For example, patients with HF with preserved EF (HFpEF) may be older and have more comorbidities than patients with HF with reduced EF (HFrEF).13 However, when compared with patients with HFpEF, those with HFrEF tend to have longer hospital stays and higher mean hospitalization costs ($8858 vs $10,286, respectively).13 However, this approach does not accurately capture the multifactorial causes of HF, and it may lead to worsened patient outcomes. Thus, a better approach to characterizing HF is needed as our understanding of the underlying pathophysiology improves.
In addition to biological considerations, other outcome metrics that include social determinants of health (SDOH) and patient-reported outcomes (PROs) could help to improve phenotyping. SDOH are potential barriers (eg, poverty, underemployment, access to care) to receiving optimal health care. By identifying and addressing these social factors, underserved populations can receive appropriate health care to manage and, hopefully, prevent acute events in HF. Distinct from statistical or clinical effects of treatment, PROs could be used to assess whether treatments lead to an actual improvement in patient quality of life (QOL) and to help guide health care providers to personalize treatment as appropriate.
Social Determinants of Health
The complexity of treating HF creates an economic, emotional, and social burden for patients and caregivers.14 This burden can be further exacerbated by SDOH that can affect the accessibility of quality health care.15 Patients with HF may also be at risk for depression and more likely to have low socioeconomic status than are individuals without HF, and they may face food insecurity or stretches of unemployment.16-20 Low socioeconomic status is associated with an increased prevalence of HF and an increased risk of death or hospitalization in patients with HF.16,18-20 In this way, SDOH impact on equity in health care, affecting the risk of HF and patients’ capacity to manage HF and, consequently, their outcomes, QOL, and well-being.18,21
By developing and supporting programs that address economic disparities, an opportunity exists to improve management of HF and prevent disease progression. Because of this wide-reaching potential impact on treatment outcomes, SDOH should be incorporated into assessments and used to inform the development of future payment programs to better assist underserved patient populations.
Patient-Reported Outcomes
In addition to objective clinical outcomes, an understanding of the patient perspective is paramount to evaluating the value and effectiveness of treatment regimens. PROs can provide data regarding symptom burden, functional limitations, and social and emotional well-being.22 These instruments are subjective, but they aim to quantify pain, functional status, or severity of disease as perceived by patients.
A prominent PRO used in patients with HF, the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12), measures physical limitations, symptoms, self-efficacy, knowledge, social interference, and overall QOL.23 Use of the KCCQ-12 improves clinicians’ accuracy in assessing health status and it leads patients to believe their clinicians better understand their symptoms.24
Innovation in Payment Reform: Refining Value-Based Payment Models
Health care policy makers continue to strive to improve quality, reduce variation, and increase the efficiency of HF care, which translates to hospitals focusing on the quality of inpatient care and developing strategies to improve care transitions.14 Traditional payment models are based on an FFS model, which can lead to fragmented, low-quality care focused on treating illness instead of supporting wellness initiatives.6 For this reason, Medicare and other payers are beginning to move away from these FFS payment models in favor of VBP models that aim to improve quality and outcomes while also reducing costs. VBP models offer increased incentives to hospitals and providers that deliver high-quality care to patients and implement strategies to address unwarranted variation.25 The use of these VBP models is increasing—in 2018, VBP models accounted for 36% of all health care spending.6
Under the 2010 Patient Protection and Affordable Care Act, the Centers for Medicare & Medicaid Services (CMS) developed several VBP programs intended to improve patient care; these included the Hospital Readmission Reduction Program (HRRP) and the Hospital Value-Based Purchasing (HVBP) Program.26
The HRRP was intended to reduce the number of patients readmitted after discharge. Beginning in 2010, the HRRP has imposed financial penalties on hospitals with high 30-day readmission rates among patients with HF; identifying the reasons why hospitals are underperforming is crucial to decrease penalties and improve quality of care. In 2019, the HRRP began using an adjusted model to compare hospitals that serve Medicare patients; this decreased penalties by 14%.26 This updated model spreads the distribution of penalties more evenly across hospitals by including SDOH in the algorithm.27 One limitation is that HRRP measures readmissions; however, deaths are not included in the program’s metrics.28
The HVBP program, a complementary program to the HRRP, provides acute care hospitals with incentive payments based on broader domains, such as patient experience, outcomes, safety, and efficiency of inpatient care for Medicare and Medicaid patients.9 This program was intended to reduce adverse events and reduce the overall costs of care.9 A limitation of the HVBP program is that it does not account for socioeconomic factors. However, results of studies assessing mortality and hospital readmissions within the HVBP program indicated no significant improvements among patients with acute myocardial infarction, HF, or pneumonia; evidence to support the effectiveness of the HVBP program is generally lacking.29-32
The Bundled Payments for Care Improvement Advanced model launched in 2018 is a voluntary alternative payment model, with HF one of the main clinical episodes included. Each clinical episode is covered from the inpatient stay to 90 days after discharge. FFS payments are issued for individual health care services during the episode and are subsequently compared against a predetermined target price. This program incentivizes high quality inpatient and outpatient care and the efficiency of care following hospital discharge.33
Barriers to Model Efficiency
Several barriers need to be addressed for health care providers and hospitals to accept VBP models. According to results of a survey of Medicaid managed care organizations, the main barrier to implementing VBP models is provider willingness to adopt alternative payment models.34 Further, models should be tailored to address the needs of rural populations with limited access to cardiologists.6 If VBP models are patient- and culture-centric, they can potentially reduce health inequalities by providing additional support to disadvantaged patient populations.6 However, more evidence is needed to demonstrate that VBP models can improve treatment outcomes and reduce costs regardless of socioeconomic status.35
Several risk assessment models that evaluate the performance of hospitals use administrative claims data, but these may not accurately capture the differences in disease severity and other geographic, social, and economic factors that can vary between hospitals.35 For example, the HRRP has penalized hospitals that serve high-risk populations, even though poor performance was likely due to the challenges in treating disadvantaged populations.36
A further challenge is that current payment models for cardiovascular conditions are mainly based on short-term episodes. This approach does not accommodate patients with HF who often need long-term care to prevent subsequent acute events. Thus, given the complexity of HF, a hybrid model that considers both long- and short-term complications would be more appropriate. This model could provide support during an episode and assist in preventing disease progression. Readmission rates in HF may be attenuated if current payment models, such as the HRRP, included additional factors from longitudinal payment models focused on specialized care.6 In addition, models that include specific population demographics and SDOH may more accurately assess hospital performance, as both social factors and health status affect treatment outcomes and health care costs.37
As a practical example, new classes of guideline-directed medical therapy (GDMT), including sodium-glucose cotransporter 2 inhibitors (SGLT2is) and angiotensin receptor-neprilysin inhibitors (ARNIs), will be key tools to incorporate into VBP models to ensure their success. Extensive literature demonstrates that the use of both SGLT2is and ARNIs in HFrEF improves survival, decreases hospitalizations, and leads to improvements in PROs.38,39 These agents may incur higher initial costs, but VBPs provide a direct incentive for quality and efficiency that can facilitate improved clinical outcomes and reduce total costs over time. Thus, there is strong evidence to support the use of new medications under new payment models. Further efforts to tailor VBP models to HF could meaningfully influence treatment outcomes and reduce overall costs.
Novel Care Delivery Approaches
Hospital at Home
To reduce readmissions in HF, hospital-at-home (HAH) interventions can provide tailored patient care at a lower cost by using nurse-led multidisciplinary teams in a patient’s home environment. Patients with HF who received at-home care, including telephone support from physicians, reported lower depression levels, improved QOL, and longer time to readmission than did patients who received hospital-based care.40,41 HAH interventions can be used to educate and provide support to patients in managing HF.
The challenges that patients experience following hospital discharge for HF can be used to inform HAH interventions.42 For instance, some patients are unable to afford required medications and need guidance in accessing discount medication programs. Furthermore, patients may need support in HF disease management overall.42 To date, most HAH interventions have occurred in small groups of patients, and larger studies are warranted to assess feasibility in real-world settings.
Digital Tools
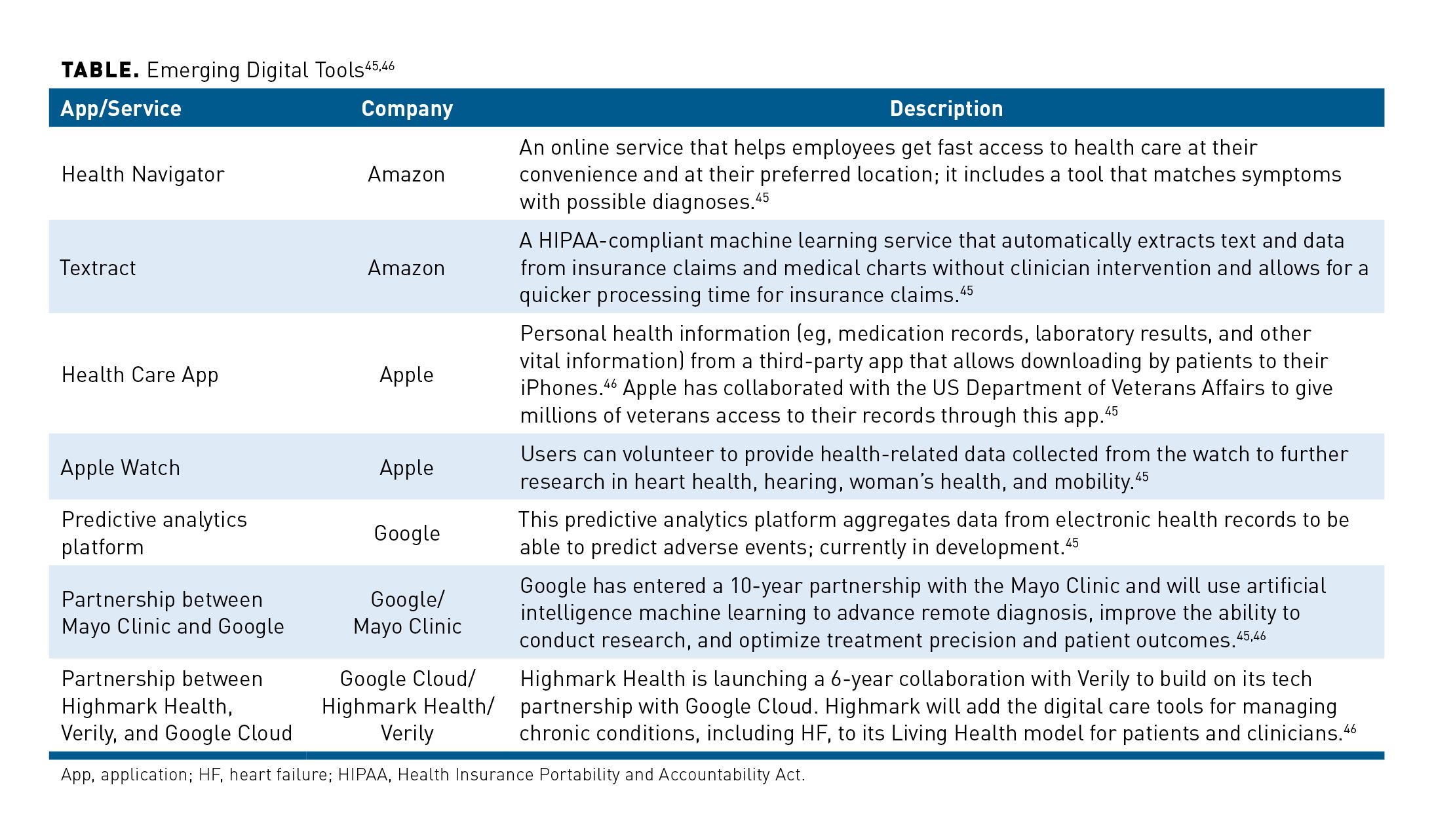
Novel innovations can foster improved quality of care and accessibility to health care services. PROMPT-HF (NCT04514458) is a recent pragmatic clinical trial that tested the effectiveness of a targeted electronic health record (EHR)-based alert system on improving the percentage of HF patients on GDMT.43 More specifically, providers were randomly assigned to receive an alert with patient characteristics and individualized GDMT recommendations vs no alert or usual care. After 30 days, higher rates of GDMT were prescribed following the alerts than what is typically observed in standard care. On the other hand, a similar EHR-based study provided clinicians with the patient’s risk of 1-year mortality during HF admissions to assess decisions about initiation or intensity of treatment, but this information did not affect hospitalizations, mortality, or clinical decision-making.44 Thus, additional studies of EHR-based tools focused on indicated treatments could be used to improve treatment decisions and overall outcomes. Other digital tools in development are described in the Table.45,46
Conclusion
HF is a growing concern that requires a multidisciplinary approach to managing the disease.47 While incumbent FFS models are based on volume, better payment models, such as VBP, have the potential to promote better short- and long-term outcomes in patients with HF by incentivizing improvements in health care.6 Further, incorporating SDOH into PROs could help to make the models more accurate in evaluating and managing HF in underserved populations.23
Continued payment reform is needed to improve treatment outcomes and reduce costs in HF and to improve health equity within the context of SDOH. By improving phenotyping and incorporating metrics that account for SDOH and PROs, new VBP models could drive improvements in treatment outcomes for patients with HF. New technologies and care delivery models could have far-reaching impacts on the current health care system.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no payment related to the development of the manuscript. Andy Shepherd, PhD, and Katie Crosslin, PhD, of Envision Pharma Group provided medical writing and editorial support, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and Lilly USA, LLC. BIPI and Lilly were given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author Affiliations: Yale School of Medicine and Yale New Haven Health (NRD, KAAC), New Haven, CT.
Funding Source: Publication of this article was supported by Boehringer Ingelheim Pharmaceuticals, Inc. and Lilly USA, LLC.
Author Disclosures: Dr Desai reports serving as a consultant or paid advisory board member for Amgen, Boehringer Ingelheim, and CSL Behring; he also reports receiving grants from AstraZeneca, Amgen, Cytokinetics, and Novartis. He has received honoraria from Bayer, Bristol Myers Squibb, and Vifor. Dr Clark reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Analysis and interpretation of data (NRD, KAAC); drafting of the manuscript (NRD, KAAC); and critical revision of the manuscript for important intellectual content (NRD, KAAC).
Address Correspondence to: Nihar R. Desai, MD, MPH. Section of Cardiovascular Medicine, Yale School of Medicine, 800 Howard Ave, New Haven, CT 06519. Email: nihar.desai@yale.edu
References
- White-Williams C, Rossi LP, Bittner VA, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Epidemiology and Prevention. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141(22):e841-e863. doi:10.1161/CIR.0000000000000767
- Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004-2016. BMC Cardiovasc Disord. 2018;18(1):74. doi:10.1186/s12872-018-0815-3
- Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014-2020). Pharmacoeconomics. 2020;38(11):1219-1236. doi:10.1007/s40273-020-00952-0
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
- Hospital Readmissions Reduction Program (HRRP). NEJM Catalyst. April 26, 2018. Accessed May 16, 2023. https://catalyst.nejm.org/doi/full/10.1056/CAT.18.0194
- Joynt Maddox K, Bleser WK, Crook HL, et al; American Heart Association Value-Based Models Learning Collaborative. Advancing value-based models for heart failure: a call to action from the value in healthcare initiative’s value-based models learning collaborative. Circ Cardiovasc Qual Outcomes. 2020;13(5):e006483. doi:10.1161/CIRCOUTCOMES.120.006483
- Reporting Options Overview. Centers for Medicare & Medicaid Services. Accessed 2022. https://qpp.cms.gov/mips/reporting-options-overview
- BPCI Advanced. Centers for Medicare & Medicaid Services. 2022. Accessed 2022. https://innovation.cms.gov/innovation-models/bpci-advanced
- Hospital Readmissions Reduction Program (HRRP). Centers for Medicare & Medicaid Services. 2022. Accessed 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
- Accountable Care Orginizations (ACOs). Centers for Medicare & Medicaid Services. Updated December 1, 2021. Accessed 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO1
- The Hospital Value-Based Purchasing (VBP) program. Centers for Medicare & Medicaid Services. Updated February 1, 2021. Accessed May 16, 2023. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HVBP/Hospital-Value-Based-Purchasing
- Wilcox JE, Yancy CW. Heart failure-a new phenotype emerges. JAMA Cardiol. 2016;1(5):507-509. doi:10.1001/jamacardio.2016.1356
- Olchanski N, Vest AR, Cohen JT, Neumann PJ, DeNofrio D. Cost comparison across heart failure patients with reduced and preserved ejection fractions: analyses of inpatient decompensated heart failure admissions. Int J Cardiol. 2018;261:103-108. doi:10.1016/j.ijcard.2018.03.024
- Piña IL, Allen LA, Desai NR. Policy and payment challenges in the postpandemic treatment of heart failure: value-based care and telehealth. J Card Fail. 2022;28(5):835-844. doi:10.1016/j.cardfail.2021.08.019
- Sterling MR, Ringel JB, Pinheiro LC, et al. Social determinants of health and 90-day mortality after hospitalization for heart failure in the REGARDS study. J Am Heart Assoc. 2020;9(9):e014836. doi:10.1161/JAHA.119.014836
- Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail. 2012;14(2):138-146. doi:10.1093/eurjhf/hfr168
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537. doi:10.1016/j.jacc.2006.06.055
- Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166-2178. doi:10.1161/CIRCULATIONAHA.117.029652
- Sun Y, Liu B, Rong S, et al. Food insecurity is associated with cardiovascular and all-cause mortality among adults in the United States. J Am Heart Assoc. 2020;9(19):e014629. doi:10.1161/JAHA.119.014629
- Witte KK, Patel PA, Walker AMN, et al. Socioeconomic deprivation and mode-specific outcomes in patients with chronic heart failure. Heart. 2018;104(12):993-998. doi:10.1136/heartjnl-2017-312539
- Social determinants of health. US Department of Health and Human Services. Accessed March 1, 2023. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
- Davis J, Olazo K, Sierra M, et al. Do patient-reported outcome measures measure up? A qualitative study to examine perceptions and experiences with heart failure proms among diverse, low-income patients. J Patient Rep Outcomes. 2022;6(1):6. doi:10.1186/s41687-022-00410-9
- Spertus JA, Jones PG. Development and validation of a short version of the Kansas City cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469-476. doi:10.1161/CIRCOUTCOMES.115.001958
- Sandhu AT, Zheng J, Kalwani NM, et al. Impact of patient-reported outcome measurement in heart failure clinic on clinician health status assessment and patient experience: a substudy of the PRO-HF trial. Circ Heart Fail. 2023;16(2):e010280. doi:10.1161/CIRCHEARTFAILURE.122.010280
- Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024
- Sukul D, Eagle KA. Value-based payment reforms in cardiovascular care: progress to date and next steps. Methodist Debakey Cardiovasc J. 2020;16(3):232-240. doi:10.14797/mdcj-16-3-232
- Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi:10.1001/jama.2018.19232
- McCarthy CP, Vaduganathan M, Patel KV, et al. Association of the new peer group-stratified method with the reclassification of penalty status in the hospital readmission reduction program. JAMA Netw Open. 2019;2(4):e192987. doi:10.1001/jamanetworkopen.2019.2987
- Banerjee S, McCormick D, Paasche-Orlow MK, Lin MY, Hanchate AD. Association between degree of exposure to the hospital value based purchasing program and 30-day mortality: experience from the first four years of Medicare’s pay-for-performance program. BMC Health Serv Res. 2019;19(1):921. doi:10.1186/s12913-019-4562-7
- Beauvais B, Whitaker Z, Kim F, Anderson B. Is the hospital value-based purchasing program associated with reduced hospital readmissions? J Multidiscip Healthc. 2022;15:1089-1099. doi:10.2147/JMDH.S358733
- Figueroa JF, Tsugawa Y, Zheng J, Orav EJ, Jha AK. Association between the value-based purchasing pay for performance program and patient mortality in US hospitals: observational study. BMJ. 2016;353:i2214. doi:10.1136/bmj.i2214
- Ryan AM, Krinsky S, Maurer KA, Dimick JB. Changes in hospital quality associated with hospital value-based purchasing. N Engl J Med. 2017;376(24):2358-2366. doi:10.1056/NEJMsa1613412
- Piña IL, Allen LA, Desai NR. Managing the economic challenges in the treatment of heart failure. BMC Cardiovasc Disord. 2021;21(1):612. doi:10.1186/s12872-021-02408-5
- Raths D. Medicaid MCO survey highlights value-based care barriers. Healthcare Innovation Group. December 9, 2020. Accessed May 16, 2023. https://www.hcinnovationgroup.com/policy-value-based-care/medicare-medicaid/article/21165920/medicaid-mco-survey-highlights-valuebased-care-barriers
- Husaini M, Joynt Maddox KE. Paying for performance improvement in quality and outcomes of cardiovascular care: challenges and prospects. Methodist Debakey Cardiovasc J. 2020;16(3):225-231. doi:10.14797/mdcj-16-3-225
- Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342-343. doi:10.1001/jama.2012.94856
- Johnson AE, Brewer LC, Echols MR, Mazimba S, Shah RU, Breathett K. Utilizing artificial intelligence to enhance health equity among patients with heart failure. Heart Fail Clin. 2022;18(2):259-273. doi:10.1016/j.hfc.2021.11.001
- Maddox TM, Januzzi JL, Jr., Allen LA, et al; Writing Committee. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772-810. doi:10.1016/j.jacc.2020.11.022
- Vaduganathan M, Claggett BL, Jhund PS, et al; Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121-128. doi:10.1016/S0140-6736(20)30748-0.
- Patel H, Shafazand M, Ekman I, Hojgard S, Swedberg K, Schaufelberger M. Home care as an option in worsening chronic heart failure -- a pilot study to evaluate feasibility, quality adjusted life years and cost-effectiveness. Eur J Heart Fail. 2008;10(7):675-681. doi:10.1016/j.ejheart.2008.05.012
- Qaddoura A, Yazdan-Ashoori P, Kabali C, et al. Efficacy of hospital at home in patients with heart failure: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0129282. doi:10.1371/journal.pone.0129282
- Sevilla-Cazes J, Ahmad FS, Bowles KH, et al. Heart failure home management challenges and reasons for readmission: a qualitative study to understand the patient’s perspective. J Gen Intern Med. 2018;33(10):1700-1707. doi:10.1007/s11606-018-4542-3
- Ghazi L, Yamamoto Y, Riello RJ, et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J Am Coll Cardiol. 2022;79(22):2203-2213. doi:10.1016/j.jacc.2022.03.338
- Ahmad T, Desai NR, Yamamoto Y, et al. Alerting clinicians to 1-year mortality risk in patients hospitalized with heart failure: the REVEAL-HF randomized clinical trial. JAMA Cardiol. 2022;7(9):905-912. doi:10.1001/jamacardio.2022.2496
- Disruption in health care. American Hospital Association. 2020. Accessed May 16, 2023. https://www.aha.org/center/emerging-issues/market-insights/disruptive-innovation/disruption-in-health-care
- Drees J. Google shakes up healthcare strategy in 2021: a timeline of key developments. Becker’s Hospital Review. December 10, 2021. Accessed May 16, 2023. https://www.beckershospitalreview.com/disruptors/google-shakes-up-healthcare-strategy-in-2021-a-timeline-of-key-developments.html
- Hessel FP. Overview of the socio-economic consequences of heart failure. Cardiovasc Diagn Ther. 2021;11(1):254-262. doi:10.21037/cdt-20-291


