- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Current and Evolving Treatment Strategies for the Alzheimer Disease Continuum
Abstract
The burden of Alzheimer disease (AD) on the US healthcare system is substantial and increasing. AD progresses along a continuum from preclinical disease characterized by normal cognition and abnormal brain biomarkers to mild cognitive impairment and then clinically apparent dementia. Diagnosis early in the AD continuum has benefits for patients and caregivers and appears cost-effective, but often, the clinical diagnosis of AD may be delayed. Currently available biomarkers include β-amyloid positron emission tomography and cerebrospinal fluid tests. Collectively, they are expensive, may lead to adverse effects, are not widely available, and are not suited for primary care. Currently available treatment options, cholinesterase inhibitors and memantine, do not alter disease progression, but can help with some symptoms. Benefits of currently available treatments on cognition are difficult to quantify and are offset by a burden of adverse effects that often go unrecognized. More accurate diagnostic biomarkers and disease-modifying drug therapies are critical unmet needs of patients with AD despite decades of clinical research. Because many phase 3 clinical trials that enrolled patients with symptomatic AD have failed, researchers believe that disease-modifying treatment is more likely to demonstrate benefit when utilized early in the disease continuum. Within the past few years, significant achievements that will advance clinical trials in early AD include the Research Framework to define and stage the AD continuum, FDA guidance on study design in early AD, and development of scales to measure cognition that are suitable for early AD. In October 2019, the AD community was re-invigorated by unexpected news that a Biologics License Application will be submitted for aducanumab to treat AD. This article explores the current state of biomarker-driven drug development across the AD continuum and reviews investigational drugs in phase 2/3 clinical development for AD.
Am J Manag Care. 2020;26:S167-S176
Introduction
Alzheimer disease (AD) is the most common cause of dementia, accounting for 60% to 80% of cases.1 It is a heterogeneous and complex disease that is challenging to differentiate from other forms of dementia, such as vascular dementia, dementia with Lewy bodies, mixed dementia, and frontotemporal dementia. Understanding of AD has evolved over the past 35 years. Before 2011, it was a clinical diagnosis that could only be confirmed by an autopsy.2 Then, diagnostic criteria were revised to allow a premortem diagnosis based on biomarkers.3,4 Now, AD is considered to be a long, degenerative process with a preclinical stage in which β-amyloid (Aβ) and tau biomarkers are present while cognition is normal, followed by neurodegeneration and a prodrome of mild cognitive impairment (MCI), which can progress to clinical AD.5
Drug therapy that changes the progression of the disease is arguably the greatest unmet need of patients with AD. Despite investing billions of dollars into clinical trials, a new AD drug has not been approved by the FDA since 2003.6,7 Drugs approved before that do not slow the progression of the disease.8 In addition, no drugs have been approved to treat MCI.9 As of May 2020, more than 100 new molecular entities were in clinical development for AD.10 Although many AD drugs have failed in late-stage clinical trials, there is hope that at least one may receive FDA approval soon. This article explores the current state of biomarker-driven drug development across the AD continuum and reviews current and emerging disease-modifying treatments for AD.
The Epidemiologic Burden of AD
The estimated number of Americans with preclinical AD based on evidence of brain Aβ is expected to rise from almost 47 million in 2017 to over 75 million by 2060.11 Modeling studies estimate that about 35% of people older than 60 years have preclinical AD.2 Because the mean time from Aβ appearance to dementia is estimated at more than 20 years, some people are likely to die of other causes before developing AD.2,12 The lifetime risk of developing AD varies according to age and disease state. For example, the lifetime risk for a 75-year-old woman is approximately 14% with no evidence of AD pathology, 24% with Aβ, 36% with Aβ and neurodegeneration; and 85% with Aβ, neurodegeneration, and MCI.12 About 15 million Americans are estimated to have MCI.13 The prevalence of MCI increases with age, with estimates of 6.7% for ages 60-64 years, 8.4% for 65-69 years, 10.2% for 70-74 years, 14.8% for 75-79 years, 25.2% for 80-84 years, and 37.6% for 85 years and older.9
Approximately 10% of Americans aged 65 years or older have AD.1 Among almost 6 million Americans 65 years or older living with AD, 80% are 75 years or older and more than 60% are women. Prevalence increases progressively with age, with AD estimates of 3% for ages 65-74, 17% for ages 75-84, and 32% for 85 years and older. By 2050, the number of Americans with AD is expected to rise to almost 14 million. The total annual cost of healthcare of patients with dementia, including long-term care and hospice, is projected to increase nearly 4-fold from $305 billion in 2020 to more than $1.1 trillion in 2050.1 A recent Centers for Disease Control and Prevention study identified 2 key factors that account for the rising prevalence of AD and other forms of dementia in the United States: a doubling in the number of Americans 65 years or older by 2060 and faster growth in minority elder populations who are disproportionately affected by dementia.14 In 2020, approximately 491,000 Americans 65 years or older are expected to receive an AD diagnosis and 700,000 are expected to die with AD. AD is the fifth leading cause of death in Americans aged 65 years or older.1
The AD Continuum
Pathophysiology
AD is characterized by brain tissue abnormalities that are both diagnostic biomarkers and targets for drug development: accumulation of Aβ plaques and oligomers outside of neurons and twisted strands of tau proteins inside neurons.1,6 In theory, Aβ accumulation contributes to neurodegeneration by interfering with neuron-to-neuron synaptic communication, whereas tau tangles may block the transport of nutrients and other essential molecules within neurons. In addition, Aβ and tau tangles may trigger microglia, which induce inflammation and are associated with abnormal glucose metabolism.15 AD is associated with distinct abnormalities in glycolysis, the main route of glucose metabolism in the brain.16 Impaired glycolysis has been correlated with more severe Aβ plaque and tau tangles postmortem.17
In 2018, the National Institute on Aging and Alzheimer’s Association developed the Alzheimer’s Diagnostic Framework to define and classify the AD continuum and distinguish it from non-AD causes of cognitive impairment based on biomarker criteria.5 The hallmark biomarkers of AD pathology—extracellular deposition of Aβ (“A”),intracellular tau tangles (“T”), and neurodegeneration (“N”)—were integrated into the “A/T/N” classification scheme to categorize subjects in AD clinical trials. The preclinical phase of the disease after Aβ deposition is classified as A+T-N-, and the transition to prodromal disease and dementia is characterized by the addition of T and N. Patients with dementia symptoms, tangles, and neurodegeneration biomarkers are classified as A+T+N- orA+T-N+.5
Apolipoprotein E (APOE) is a susceptibility gene for AD. The APOE4 allele is linked to a higher risk of AD, while the APOE2 allele is associated with a lower risk of AD. In a recent case-control study in more than 5000 people, the odds ratio for developing AD with the APOE2/2 genotype was 66% less than the APOE2/3 genotype, 87% less than the APOE3/3 genotype, and 99.6% less than the APOE4/4 genotype.18 Because the relative risk conferred by APOE is impacted by gender and other genetic and environmental factors, the APOE4 allele is neither necessary nor sufficient to cause AD. APOE genotyping for AD risk prediction is not recommended because of limited clinical utility and poor predictive value.19 The APOE allele is a consideration in clinical trials of monoclonal antibodies and other drugs that target Aβ plaque because they can cause amyloid-related imaging abnormalities (ARIA) that can be accompanied by adverse effects (AEs).20,21
Disease Course
AD progresses along a continuum with 3 phases: preclinical disease, MCI, and clinically apparent dementia.1,5,22 Although both cognitive decline and biomarker measurements progress over time, biomarker progression begins before symptom onset.5
Preclinical AD
The preclinical phase of the AD continuum is characterized by Aβ pathology that can be detected by neuroimaging and cerebrospinal fluid (CSF) biomarkers in individuals who are not cognitively impaired.5 In longitudinal observational studies, brain accumulation of Aβ in cognitively normal individuals has been linked to a greater risk of progression to MCI and dementia.23-25 Although preclinical AD is considered asymptomatic, screening data from the Anti-Amyloid Treatment in Asymptomatic Alzheimer Disease (A4) Study recently showed that participants with elevated Aβ had lower Preclinical Alzheimer Cognitive Composite scores and more reports of subtle recent declines in daily cognitive function.26 It is important to bear in mind that the presence of Aβ plaque does not necessarily predict the development of dementia.1
MCI
In addition to the presence of biomarkers, patients with MCI have subtle problems with memory and thinking that may not be readily apparent or interfere with the ability to perform activities of daily living (ADLs).1 MCI can improve, remain stable, or progress to AD or other conditions. In observational studies, 32% to 38% of patients with MCI progressed to dementia over 5 years of follow-up.27,28 The American Academy of Neurology recommends evaluating patients for MCI if they or a close contact raise concerns about memory or impaired cognition.9 Early diagnosis gives patients and significant others more time to address finances and estate planning, take steps to prevent exploitation, plan for care, and address driving safety issues.29 Early diagnosis can also improve healthcare delivery.29 For example, patients may need help from a family member and written instructions to maintain medication adherence. Color-coded weekly or monthly adherence packaging prepared by the pharmacy can also help. Drugs with cognitive AEs that can exacerbate MCI can be avoided. Patients with MCI should be periodically monitored for changes in cognitive status.9 Documenting the diagnosis in health records alerts other clinicians and may reduce mismanagement.
Alzheimer Dementia
AD severity is characterized as mild, moderate, or severe based primarily on the ability to perform ADLs. In mild disease, most patients are able to function independently but are likely to require assistance. These patients may still be able to drive and work. In moderate AD, patients may have difficulties communicating and performing ADLs, such as bathing and dressing. They may exhibit personality and behavior changes. In severe AD, patients need substantial assistance with ADLs and likely require around-the-clock care. These patients may become bedridden due to damage to areas of the brain involved in movement. They may have difficulty swallowing, which can lead to aspiration pneumonia, a contributing cause of death among many patients with AD. In people aged 65 years or older who receive a diagnosis of AD, mean survival is 4 to 8 years, but can reach 20 years.1 A person living with AD between age 70 and 80 years is expected to have severe dementia for 4 years and reside in a nursing home for most of that time.1 About 40% of patients with AD have severe dementia that requires care on par with that provided in a nursing home.11
Diagnosis
Despite the need for early and accurate diagnosis, an estimated 29% to 76% of patients with dementia in primary care are undiagnosed.30 Clinical criteria for AD include a history of cognitive decline with a gradual onset and progressive course, exclusion of other causes, and documented cognitive impairment in at least one domain (complex attention, executive function, learning and memory, language, perceptual motor function, and social cognition).1 The diagnosis should be based on a thorough cognitive and neurologic examination and ideally should include history from close contacts about the patient’s cognitive status.1 The diagnostic evaluation may also include blood tests and magnetic resonance imaging to document neurodegeneration and rule out other forms of dementia and neurodegenerative diseases. Batteries of neuropsychological testing may have value, including to distinguish between dementia subtypes, but they are time consuming and may not be widely available.31
Although it has been part of the Medicare Annual Wellness Visit since 2011, a recent Alzheimer’s Association survey found that just 47% of primary care physicians routinely assess older patients for cognitive impairment.1 Evaluation should include direct observation of cognitive function and may include a brief validated, structured cognitive assessment tool. A positive cognitive screening test should lead to additional diagnostic testing to confirm the diagnosis and determine the dementia subtype.30 Of note, a 2020 US Preventive Services Task Force panel found insufficient evidence to recommend for or against routine cognitive impairment screening in asymptomatic community-dwelling adults 65 years old and older.30
Cognitive Screening Tests
Many standardized mental status scales are used to document the presence and progression of dementia, including the Montreal Cognitive Assessment and the Mini-Mental State Examination (MMSE). Although these scales can accurately differentiate clinically apparent Alzheimer-type disease (CATD) from normal cognition, they are less accurate in distinguishing mild CATD versus normal cognition and CATD versus MCI.32
Biomarkers
More accurate diagnostic biomarkers are a critical unmet need because 15% to 30% of patients with CATD do not meet postmortem diagnostic criteria for AD.1 Current diagnostic biomarkers that differentiate AD from other forms of dementia include brain imaging and CSF biomarkers. FDA-approved Aβ positron emission tomography (PET) scanning agents include florbetapir, flutemetamol, and florbetaben. The Alzheimer’s Association and Society of Nuclear Medicine and Molecular Imaging recommend use by dementia experts evaluating patients with cognitive impairment if scan results will improve diagnostic certainty and alter the treatment plan.33 A recent Agency for Healthcare Research and Quality systematic review found that Aβ PET was highly sensitive and specific for Aβ pathology of AD and may increase classification accuracy.31 In clinical trials evaluating disease-modifying drugs for AD, biomarkers play a crucial role in identifying eligible patients and contribute to the high cost of AD clinical trials. The out-of-pocket cost per scan is at least $3000.34 Although commercial insurance coverage varies, Medicare does not cover them unless they are conducted as part of a clinical trial assessing whether Aβ imaging improves patient outcomes or advances patient treatment options.34
The Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study is evaluating whether Aβ PET scanning affects the management and outcomes of dementia care in Medicare beneficiaries.35 In the initial phase of the study, physicians changed treatment based on scan results in greater than 60% of patients. More than a third of patients who were presumed to have AD had a negative PET scan, whereas more than half of patients presumed not to have AD had a positive PET scan. In patients with a positive PET scan, AD drug prescribing increased from 40% to 82% in patients with MCI (P <.001), although no drug has established efficacy to treat MCI.9Prescribing increased from 63% to 91% in patients with dementia (P <.001). AD drug prescribing decreased modestly in patients with a negative scan.35
The first diagnostic imaging agent that identifies AD-associated tau pathology may be available soon. The FDA is considering approval of flortaucipir, a PET scanning tracer that binds with tau tangles.36 In a phase 3 postmortem validation study, it had sensitivity of 92% to 100% and specificity of 52% to 92% for predicting tau pathology.37
PET and CSF biomarkers for Aβ are invasive, expensive, time consuming, cause AEs, and are not widely available.13,34 Biomarkers in clinical development may address these limitations. For example, a blood test for plasma phosphorylated-tau181 predicted tau and Aβ pathologies, identified AD across the clinical continuum, and differentiated it from other neurodegenerative diseases in validation studies.38,39
AD Management Considerations
Public Health Burden
AD is a substantial public health burden because patients are disabled and dependent on others for care for a substantial portion of the disease.1 The disease burden of AD has increased dramatically in the United States over the past few decades. Disability-adjusted life-years (DALYs), the primary measure of disease burden, are calculated by adding the number of years of life lost due to premature mortality (YLLs) and the number of years lived with disability (YLDs) caused by a disease. Based on DALYs, AD rose in the ranking of burdensome diseases from twelfth in 1990 to sixth in 2016. In 2016, it ranked fourth for YLLs and nineteenth for YLDs.1
Value of Early Diagnosis
A recent study commissioned by the Alzheimer’s Association estimated the potential cost savings of early diagnosis.1 Diagnosis in the MCI phase rather than in the dementia phase or not at all could save approximately $7 trillion in medical and long-term care costs. Cost savings would accrue from a lower cost to diagnose MCI versus dementia and lower medical and long-term care costs for diagnosed and managed MCI and dementia versus unmanaged MCI and dementia.1 Unfortunately,many barriers exist to early diagnosis of MCI in primary care, including a lack of assessment tools, training, time, and infrastructure.40
Comorbidities
Medicare beneficiaries with dementia have a higher rate of comorbidities than those without dementia.1 For example, the prevalence of more than 5 chronic conditions is 26% and 4%, respectively. In 2014, among Medicare beneficiaries with dementia, 38% had comorbid coronary artery disease, 37% had diabetes, 29% had chronic kidney disease, 28% had congestive heart failure, and 25% had chronic obstructive pulmonary disease.1
Caregiver Burden
As the US population ages, the proportion of younger adults available to provide care for patients with AD is expected to decline.14 Whereas the ratio of potential younger caregivers to older high-risk adults is currently 7 to 1, it is expected to decline to 4 to 1 by 2030. Negative impacts on caregivers include greater work instability, lower household income and personal savings, increased food insecurity, and less personal medical care. This leads to caregiver health problems, higher rates of depression and psychological stress, and even a higher risk of mortality.1,14 In 2017, Medicare implemented a billing code to reimburse services related to care planning and coordination for patients with cognitive impairment and their caregivers. This is intended to provide greater caregiver support.1
The COVID-19 pandemic has created a cascade of negative effects for patients with dementia and their caregivers. It has amplified the burden of caregiving for patients with dementia. Mortality from COVID-19 is extremely high in patients with dementia due to advanced age, comorbidities, and difficulty complying with safeguarding procedures such as wearing masks, handwashing, and social distancing.41,42 COVID-19 outbreaks in nursing homes have high attack and case fatality rates.42,43 Whereas nursing home residents and workers account for 11% of COVID-19 cases in the United States, they account for 35% of deaths.43 Given this risk, facilities have implemented numerous restrictions. For patients who are cared for at home, social distancing measures and the loss of in-home health aides and adult day care has placed the entire burden of caregiving on immediate family members.41 Caregivers, whether at home or in nursing homes, are experiencing extreme stress and social isolation during the pandemic.41,42
Current Management of the AD Continuum
Patients on the AD continuum often take medications that impair cognition and may accelerate the disease trajectory, including benzodiazepines, anticholinergics, and other psychotropic drugs.44-46 Because “Do no harm” is a guiding precept in elder care, avoiding these drugs is a crucial strategy to preserve cognition.47 The Beers list of potentially inappropriate medications acts as a guide to avoiding harmful prescribing in patients with AD.48 Given that medication nonadherence is endemic in patients with AD, it is important to simplify drug regimens and deprescribe unnecessary drugs.49 Factors linked to medical nonadherence in patients with dementia include taking more than 4 drugs, use of an anticholinergic, and a pill burden.49 Caregivers should receive medication management support, with periodic reassessment of the patient’s medication needs as AD progresses.50 A randomized clinical trial is currently evaluating whether deprescribing inappropriate medications and optimizing medication regimens can delay the onset of symptomatic AD and other forms of dementia.51
Nonpharmacologic Management of AD
A few nondrug modalities have been shown to improve cognition in patients with AD. Exercise has positive effects on cognitive function, and may even slow the rate of cognitive decline in patients with AD.1 Cognitive stimulation has been found to improve cognitive function, depression, anxiety and quality of life (QOL) in patients with AD. Cognitive training may improve overall cognition that may last for at least a few months in patients with mild to moderate dementia.1 Nondrug modalities also play an important role in reducing behavioral symptoms, such as sleep disturbances, wandering, depression, agitation, and aggression. Nonpharmacologic interventions for agitation and aggression avoid the potential harms of antipsychotics and may outperform pharmacologic approaches. For example, outdoor activities were found to be more efficacious than antipsychotics for managing physical aggression.52 Other beneficial modalities for agitation include multidisciplinary care planning, pet therapy, music, and massage.52
Pharmacologic Management of AD
As shown in Table 1, FDA-approved drugs for AD include cholinesterase inhibitors and memantine.53 Cholinesterase inhibitors increase levels of the neurotransmitter acetylcholine while memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist that affects glutamatergic transmission. Memantine is used alone or in combination with a cholinesterase inhibitor. Each of these medications is taken orally, once or twice daily. Rivastigmine is also available as a transdermal patch that is applied once daily.53
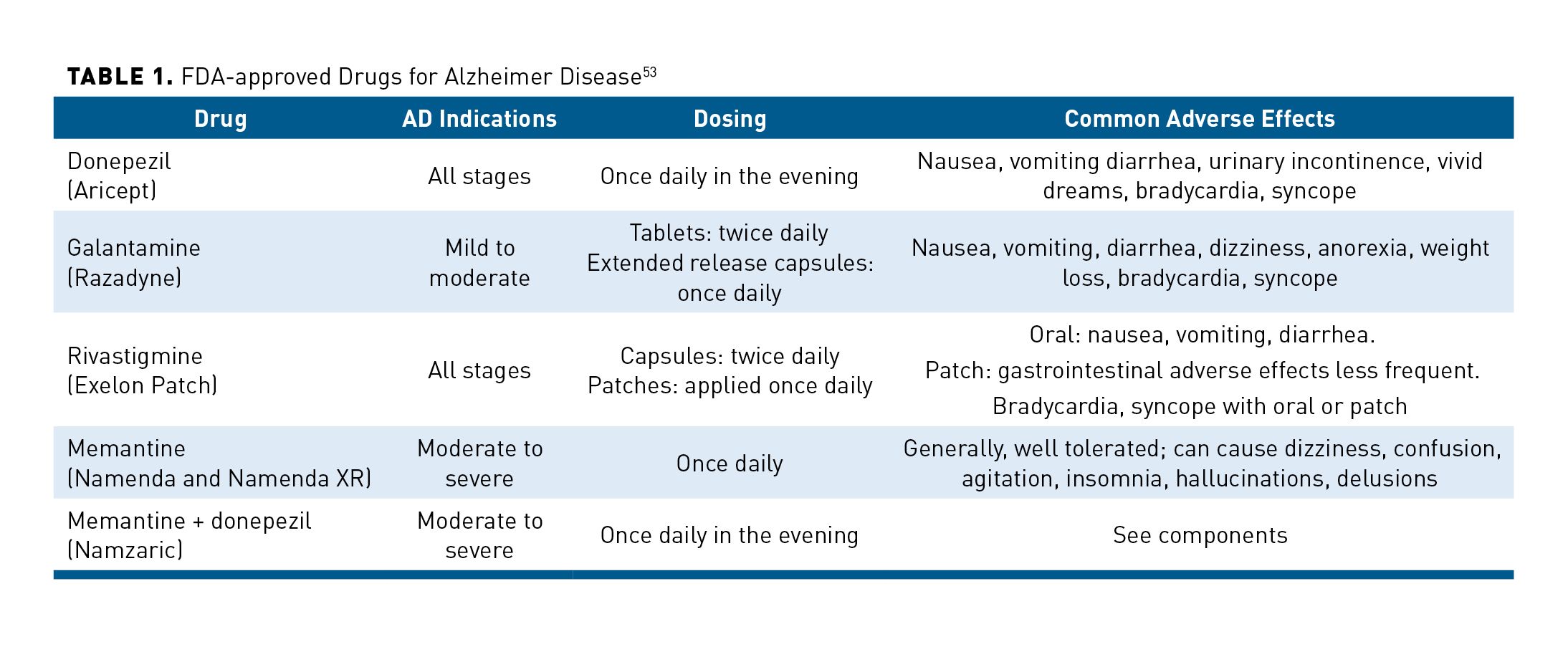
Cholinesterase inhibitors and memantine do not change the progression of AD. In the 1-year AD2000 clinical trial, donepezil demonstrated slight improvements in cognition and function, but failed to delay institutionalization, reduce caregiver burden, or lower costs.54 Results of a recent Cochrane review of donepezil trials showed a mean reduction of -2.7 points on the Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog, range 0-70), but no benefit for behavioral symptoms, QOL, or total healthcare resource utilization.55 This falls below the 4-point difference on the ADAS-Cog that is generally recognized as clinically meaningful.56 AEs with donepezil were dose related. In particular, donepezil 23 mg/day did not improve cognitive function more than 10 mg/day, but increased the risk of AEs and premature treatment discontinuation.55 In another recent Cochrane review, memantine demonstrated a small clinical benefit versus placebo in patients with moderate to severe AD, but had no benefit in patients with mild AD.57
In a systematic review that excluded clinical trials with a high risk of bias, cholinesterase inhibitors and memantine slightly reduced short-term cognitive decline.8 It was unclear if these effects are clinically meaningful. Cholinesterase inhibitors had a small mean improvement in cognition with a median standardized mean difference (SMD) of 0.30 (range, 0.24, 0.52). For function, effects ranged from no difference to a small improvement, with a median SMD of 0.19 (range, -0.10-0.22). Memantine did not demonstrate any benefit in patients with mild to moderate CATD. In moderate to severe CATD, insufficient to low-strength evidence inconsistently suggested that adding memantine to a cholinesterase inhibitor improved cognition, but not function.8
As part of the Choose Wisely initiative, the American Geriatrics Society recommends against prescribing cholinesterase inhibitors unless cognitive benefits and gastrointestinal AEs are monitored.58 A cholinesterase inhibitor should be stopped after 12 weeks if improvements are not observed on practical treatment outcomes reflecting stabilization of cognition that can be easily assessed. Patients and caregivers should be counseled about AEs before beginning a trial of cholinesterase inhibitors. Deprescribing of cholinesterase inhibitors has been linked to a reduction in falls and fractures, but not an increase in the risk of aggressive behaviors and antipsychotic prescribing in nursing home residents with severe dementia.59,60 Cholinesterase inhibitor AEs are often unrecognized and lead to a “prescribing cascade” that contributes to a treatment burden in patients with AD. For example, observational studies have found that patients receiving a cholinesterase inhibitor had an increased risk of receiving an anticholinergic drug to manage urinary incontinence, which is likely caused or exacerbated by the cholinesterase inhibitor.61,62
Treatment Landscape of Emerging Agents for the AD Continuum
Since 2007, more than 50 drugs have failed in phase 3 clinical trials for AD.63 This includes Aβ-targeting agents (bapineuzumab, crenezumab, semagacestat, solanezumab), β-secretase inhibitors (elenbecestat, lanabecestat, umibecestat, verubecestat), intravenous immunoglobulin, aspirin, ginkgo biloba, idalopirdine, minocycline, nivaldipine, pioglitazone, and others.6,64-77 Because these failed studies enrolled patients with symptomatic AD, many researchers now believe that clinical trials of disease-modifying drugs should be conducted much earlier, either during the preclinical or MCI phases of the AD continuum.1 In 2018, the FDA published a draft guidance for drug development in early AD that aligns with this thinking.78 As shown in Table 2, it does away with the preclinical/prodromal definition in favor of 3 stages leading to early AD.78 It now refers to a “persistent effect on disease course” instead of a disease-modifying effect.79
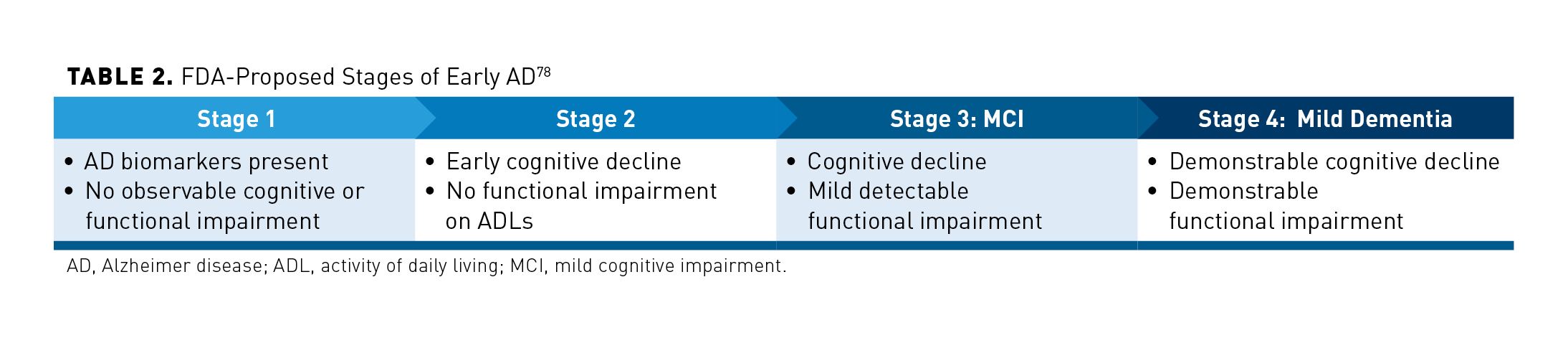
Many factors may have contributed to the high failure rate of AD phase 3 trials. Because AD is a heterogeneous disease, the inaccuracy of disease biomarkers is a likely factor.7 In hindsight, some trials may have failed because dose response was poorly understood.80 Now experts assert that before advancing to phase 3 trials, a drug should demonstrate sufficient brain exposure, strong evidence of target engagement, a clear dose response, and a large effect size in phase 2 trials.21,80,81 Target engagement is a hot-button issue for investigational anti-Aβ monoclonal antibodies. Some experts theorize that some have failed because they target amyloid monomers or insoluble fibrils or plaques rather than soluble Aβ oligomers, which may be a key mediator of neurodegeneration.21 Coprimary study end points of cognition and function that were required by the FDA may have also contributed by setting an unachievable standard for efficacy.7 The draft guidance now suggests an AD biomarker and a cognitive or functional end point could be used to demonstrate efficacy in the earliest stages of AD (FDA stage 1 and 2).7 It also signals that the agency is willing to grant accelerated approval based on biomarker data with a requirement that a confirmatory clinical trial demonstrates a delay in symptom onset.7
Up to now, clinical trials have used the ADAS-cog as an efficacy end point, but it is not very sensitive or reliable in mild dementia.7 For patients in FDA stage 3 criteria (MCI), the draft guidance suggests an integrated scale that assesses both function and cognitive effects could serve as a single primary efficacy end point. New instruments, such as the integrated AD rating scale or AD Composite Score (ADCOMS), combine cognitive and functional outcomes. ADCOMS combines portions of the ADAS-cog, Clinical Dementia Rating (CDR) scale, and MMSE that have been shown to change the most over time in people who do not have functional impairment yet.7 As of May 2020, there were 13 drugs in phase 2/3 clinical development for AD.10,82 They target Aβ, tau, inflammation, neuroprotection, and metabolism.75
Aβ-Targeting Investigational Drugs
Aducanumab
This fully-human IgG1 monoclonal antibody binds selectively to aggregated Aβ fibrils and soluble oligomers.83,84 In March 2019, after an interim futility analysis predicted the phase 3 placebo-controlled EMERGE and ENGAGE trials would not meet their primary end points, the termination of all aducanumab clinical trials was announced. However, in a subsequent analysis of a larger data set from the EMERGE trial, aducanumab met the primary end point, the Clinical Dementia Rating–Sum of Boxes (CDR-SB) score after 18 months of treatment.85 The highest dose (10 mg/kg) demonstrated a 23% reduction in the CDR-SB (P = .01). This translates to an absolute change of –0.4 on an 18-point scale. The high-dose group also declined less on secondary end points, including the ADAS-Cog (–27%; P = .01) and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living scale (ADCS-ADL-MCI; –40%; P = .001). Although the ENGAGE trial did not meet the primary end point, an exploratory analysis suggested slower decline in patients who received at least 10 doses of the highest aducanumab dose. In sub-studies of EMERGE and ENGAGE, aducanumab caused a dose-dependent reduction in Aβ and some reduction in CSF phosphorylated-tau.85
Amyloid-related imaging abnormalities-edema (ARIA-E), the most common AE with aducanumab, occurred in 35% of patients; 74% of cases were asymptomatic and episodes generally resolved within 4 to 16 weeks.85 In the EMERGE trial, the rate of permanent treatment discontinuation for an AE was 2.9% with placebo versus 7.7% with low-dose and 8.8% with high-dose aducanumab.86 Respective discontinuation rates because of ARIA were 0.2%, 4.6%, and 6.6%.86
What accounts for these divergent results? The initial analysis included about half as many patients as the final data set and was limited to patients who had completed the study by the end of 2018. These patients, early enrollers in the studies, had lower mean aducanumab exposure. The futility analysis was not adjusted to consider the effect of 2 late protocol amendments that led to more patients receiving the highest dose of aducanumab later in the study.87 Specifically, these protocol amendments increased the dose in APOE4 carriers who are more susceptible to ARIA after data from other studies suggested ARIA is manageable and usually resolves without sequelae. In early July 2020, the manufacturer completed its submission of a Biologics License Application for aducanumab as a treatment for AD.88,89
BAN2401
This humanized monoclonal antibody has greater affinity for soluble Aβ protofibrils (ie, large amyloid oligomers) than insoluble fibrils or plaques.20 In Study 201, a phase 2b trial, there was a significant dose-dependent reduction in Ab PET burden across all doses in 856 patients with MCI or early AD. At 18 months, 81% of participants on the highest dose had Aβ-negative scans. There was a dose-dependent reduction in cognitive decline on the ADCOMS, starting at 6 months. The highest dose had a 30% reduction in the ADCOM versus placebo (P = .034). Dose-related ARIA-E occurred in fewer than 10% of patients and was more frequent in APOE4-positive patients. It generally occurred within the first 3 months of treatment, resolved within 4 to 12 weeks, and caused headache or confusion in about 10% of cases.20
Based on Study 201 results, the phase 3 CLARITY trial is examining BAN2401 in more than 1500 patients with MCI due to AD or mild AD with confirmed Aβ pathology.90 In this study, BAN2401 will be given at a dose of 10 mg/kg every other week. The primary end point is the change from baseline in the CDR-SB after 18 months of treatment. Initial results are expected in 2022.90 BAN2401 is also being evaluated for the prevention of AD in the placebo-controlled phase 3 AHEAD 3-45 trial.91,92 The trial consists of 2 sub-studies: A3 and A45. The A3 sub-study will evaluate low-dose BAN2401 in cognitively normal individuals who are currently below the threshold for Aβ elevation on Aβ PET scanning but are at high risk for further Aβ accumulation. The A45 sub-study will evaluate high-dose BAN2401 in clinically normal participants (ie, little to no cognitive impairment) with elevated levels of Aβand are at high risk for progression to MCI and AD dementia.91,92
Gantenerumab
This IgG1 human monoclonal antibody binds to aggregated forms of Aβ and has been shown to reduce the burden of Aβ plaque in patients with AD.63,93 Two ongoing phase 3 clinical trials, GRADUATE 1 and 2, are assessing subcutaneous (SC) gantenerumab for the treatment of early AD in more than 2000 patients. Gantenerumab is the only anti-Aβ agent in late-stage clinical trials that is administered by SC injection. In February 2020, it was announced that gantenerumab did not meet its primary end point in the DIAN-TU trial, an international clinical trial evaluating multiple drugs in patients with or at risk for autosomal dominant AD. The GRADUATE studies are evaluating a higher gantenerumab dose that most patients received in the DIAN-TU trial. Results are expected in 2022.93
Tau-targeting Investigational Drugs
LMTX
This prodrug of methylene blue, a second-generation tau aggregation inhibitor, is the only tau-specific agent in phase 3 clinical trials.6,94 Blinding of LMTX in clinical trials is problematic because it discolors urine. Two phase 3 clinical trials of LMTX for mild to moderate AD (TRX-005 and TRX-015) were completed in 2016. Based on phase 2 clinical trial results, these studies compared LMTX doses of 150-250 mg/day with 8 mg/day as an intended control that would maintain blinding. In TRX-015, 8 mg/day appeared to show the same benefit as the higher doses, and LMTX monotherapy appeared more effective than coadministration with cholinesterase inhibitors and/or memantine. These findings led to changes in the design of the ongoing TRX-005 study. That study met modified primary prespecified outcomes and confirmed the results seen in Study TRx-015.95 A third phase 3 clinical trial, LUCIDITY, is currently underway to confirm whether low-dose monotherapy is effective.96 Top-line results are expected by the end of 2021. To maintain blinding, a urine discolorant has been added to some placebo pills in this study.96 AEs with LMTX included diarrhea, dysuria, and decreased hemoglobin. It does not appear to cause ARIA.6
Inflammation-targeting Investigational Drugs
ALZT-OP1
This combination regimen consists of nasally inhaled cromolyn, a mast cell stabilizer, and oral ibuprofen, a nonsteroidal anti-inflammatory agent. In theory, it could reduce neuroinflammation and promote clearance of Aβ by converting microglia from a proinflammatory to a phagocytic state.94 The phase 3 COGNITE trial is evaluating whether ALZT-OP1 slows or reverses cognitive and functional decline on the CDR-SB in patients with early AD.97
COR388
This oral small-molecule agent irreversibly inhibits gingipains.98 These are virulence factor proteases from Porphyromonas gingivalis, a periodontal pathogen that has been found in the brain of patients with AD. COR388 is being evaluated in the phase 2/3 GAIN clinical trial.99 It is evaluating COR388 given twice daily in 570 patients with mild to moderate AD. An interim analysis is expected by the end of 2020, and top-line results are expected by the end of 2021.99
Masitinib
This selective tyrosine kinase inhibitor acts on mast cells and modulates neuroinflammation.100 Potential mechanisms of action in AD include modulating neuroinflammation mediated by mast cells and inhibition of Fyn, a protein kinase involved in Aβ signaling and tau phosphorylation. In June 2019, interim analysis of the phase 3 AB09004 clinical trial indicated that the study should continue. The study is comparing masitinib 4.5 or 6 mg/kg/day with placebo in 720 patients with confirmed mild to moderate AD receiving a cholinesterase inhibitor and/or memantine.100 The study is expected to complete in 2020.101
Other Investigational Drugs
AGB101
Levetiracetam is being repurposed as a synaptic vesicle glycoprotein 2A (SV2A) modulator that is hypothesized to decrease Aβ-induced hippocampal hyperactivity.75 The phase 3 HOPE4MCI clinical trial is evaluating low-dose extended-release levetiracetam at a dose 220 mg once daily to slow cognitive and functional impairment, as in patients with MCI.102 The primary outcome is change in the CDR-SB. Top-line results are expected in September 2022.102
Blarcamesine (ANAVEX2-73)
This sigma-1 receptor agonist targets protein misfolding, and may reduce tau hyperphosphorylation, oxidative stress, and neurodegeneration in AD.103 In a phase 2a study, it demonstrated dose-dependent improvement in MMSE ADCS-ADL. A 48-week phase 2b/3 study in 450 patients with early AD is ongoing. Top-line results are expected in September 2023.104
CAD106
This second-generation active Aβ vaccine contains multiple copies of the Aβ1–6 peptide linked to a carrier containing many copies of bacteriophage Qb coat protein.105 In animal studies, it induced Aβ antibodies without triggering an Aβ-specific T-cell response. It is under evaluation in a 5-year phase 2/3 trial in individuals aged 60 to 75 years who are asymptomatic APOE4 carriers with elevated Aβ in CSF or on PET scan.106 CAD106 is given by intramuscular injection every 6 weeks for the first 3 injections and then every 3 months thereafter. The CAD106 trial is expected to yield top-line results by the end of 2024.107 In a phase 2b clinical trial, serious AEs more frequent with CAD106 than placebo included headache, hypertension, and fever. Several cases of ARIA occurred in CAD106-treated patients.105
Icosapent Ethyl
The phase 2/3 BRAVE-EPA study is evaluating the effect of icosapent ethyl in 150 cognitively-normal APOE4-positive veterans aged 50 to 75 years. Initial results are expected in late 2021.108
Plasma Exchange
The phase 2/3 Alzheimer’s Management by Albumin Replacement (AMBAR) trial assessed whether plasma exchange and infusions ofalbumin ± intravenous immunoglobulin (IVIG) would delay progression of mild to moderate AD.109 Plasma exchange may remove albumin-bound Aβ circulating in plasma. Albumin and IVIG have immunomodulatory and anti-inflammatory properties. In top-line data, plasma exchange did not significantly affect the coprimary end point of ADAS-Cog and ADCS-ADL scores compared with placebo at 14 months but had significant benefits on some individual end points. The sponsor intends to meet with the FDA to discuss the design of a successive AMBAR IItrial to confirm whether the protocol has benefit.110 The feasibility of plasma exchange, an invasive and expensive procedure, to manage progression of AD is questionable.111
Troriluzole (BHV-4157)
This once-daily oral drug is a prodrug conjugate of riluzole that may reduce glutamate-mediated excitotoxicity and nerve cell deterioration by promoting nerve cell glutamate reuptake.75 It is being evaluated in the T2 Protect AD phase 3 clinical trial, which could have preliminary results by late 2020.112
The Future of AD Drug Development
More than 1000 clinical trials have been put on hold because of the COVID-19 pandemic.113 This includes clinical trials in AD that have enrolled participants who are especially vulnerable to COVID-19 complications.114 Researchers are concerned that data collection in ongoing AD clinical trials may be harmed. Because conducting clinical trials in AD is already a daunting undertaking, there is also concern that some pharmaceutical companies may abandon a challenging drug development area such as AD in this time of crisis.42
What does the future hold if a drug receives FDA approval to prevent progression of preclinical or prodromal AD? A RAND Corporation analysis suggests that a paradigm shift to preventing progression in people with preclinical or prodromal disease could create enormous challenges for the US healthcare system, especially managed care.13 Necessary resources such as dementia specialists, PET imaging facilities, and infusion centers are in short supply. Between 2020 and 2040, 2.1 million patients might develop AD while on a waiting list for treatment.13Considering the large number of individuals with preclinical AD, the cost of preventive therapy could be enormous.115 Assessing the value of treatment will likely be a complex undertaking if a drug is approved based on a surrogate measure (eg, cognitive test score improvement) that does not guarantee prevention of dementia. For the first time in almost 20 years, the FDA is evaluating whether to approve a drug for AD that could modify its course. This exciting news has broad implications in managed care.
Author affiliation: Richard A. Marasco, BS Pharm, FASCP, BCGP, HRM, is a clinical instructor of pharmacy practice, Philadelphia College of Osteopathic Medicine, Mercer University College of Pharmacy, South University College of Pharmacy, Quitman, GA.
Funding source: This activity is supported by an educational grant from Biogen MA, Inc.
Author disclosure: Dr Marasco has no relevant financial relationships with commercial interests to disclose.
Authorship information: Substantial contributions to the concept and design; drafting of the manuscript; overall supervision; and critical revision of the manuscript for important intellectual content.
Address correspondence to: rmarasco@seniorpharm.com
Medical writing and editorial support: Jill E. Allen, PharmD, BCPS
REFERENCES
1. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):1-391.
2. Mar J, Soto-Gordoa M, Arrospide A, Moreno-Izco F, Martínez-Lage P. Fitting the epidemiology and neuropathology of the early stages of Alzheimer’s disease to prevent dementia. Alzheimers Res Ther. 2015;7(1):2. doi: 10.1186/s13195-014-0079-9
3. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging−Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008
5. Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018
6. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. doi: 10.1177/1179573520907397
7. Sabbagh MN, Hendrix S, Harrison JE. FDA position statement “Early Alzheimer’s disease: Developing drugs for treatment, Guidance for Industry”. Alzheimers Dement (N Y). 2019;5:13-19. doi: 10.1016/j.trci.2018.11.004
8. Fink HA, Linskens EJ, MacDonald R, et al. Benefits and harms of prescription drugs and supplements for treatment of clinical Alzheimer-type dementia: a systematic review and meta-analysis. Ann Intern Med. 2020;172(10):656-668. doi: 10.7326/M19-3887
9. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi: 10.1212/WNL.0000000000004826
10. Alzforum. Therapeutics search. Published 2020. Accessed May 12, 2020. alzforum.org/therapeutics/search?fda_statuses%5B%5D=181&fda_statuses%5B%5D=33251&fda_statuses%5B%5D=182&fda_statuses%5B%5D=931&fda_statuses%5B%5D=183&target_types=&therapy_types=&conditions%5B%5D=145&conditions%5B%5D=146&keywords-entry=&keywords=#results
11. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121-129. doi: 10.1016/j.jalz.2017.10.009
12. Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018;14(8):981-988. doi: 10.1016/j.jalz.2018.03.005
13. Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the preparedness of the U.S. health care system infrastructure for an Alzheimer’s treatment. RAND Corporation. Published 2017. Accessed May 10, 2020. rand.org/pubs/research_reports/RR2272.html
14. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged≥65 years. Alzheimers Dement. 2019;15(1):17-24. doi: 10.1016/j.jalz.2018.06.3063
15. Hampel H, Caraci F, Cuello AC, et al. A path toward precision medicine for neuroinflammatory mechanisms in Alzheimer’s disease. Front Immunol. 2020;11:456. doi: 10.3389/fimmu.2020.00456
16. Johnson EC, Dammer EB, Duong DM, et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020;26(5):769-780. doi: 10.1038/s41591-020-0815-6.
17. An Y, Varma VR, Varma S, et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14(3):318-329. doi: 10.1016/j.jalz.2017.09.011
18. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al; Alzheimer’s Disease Genetics Consortium. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020;11(1):667. doi: 10.1038/s41467-019-14279-8
19. Choosing Wisely. American College of Medical Genetics and Genomics. Updated September 15, 2016. Accessed July 21, 2020. choosingwisely.org/clinician-lists/american-college-medical-genetics-genomics-apoe-genetic-testing-to-predict-alzheimer-disease/
20. Selkoe DJ. Light at the end of the amyloid tunnel. Biochemistry. 2018;57(41):5921-5922. doi: 10.1021/acs.biochem.8b00985
21. Tolar M, Abushakra S, Sabbagh M. The path forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement. Published online January 3, 2020. doi: 10.1016/j.jalz.2019.09.075
22. American Geriatrics Society. A guide to dementia diagnosis and treatment. Published 2011. Accessed July 21, 2020. unmfm.pbworks.com/f/American+Geriatric+Society+Dementia+Diagnosis+03-09-11.pdf
23. Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957-965. doi: 10.1016/S1474-4422(13)70194-7
24. Amariglio RE, Buckley RF, Mormino EC, et al. Amyloid-associated increases in longitudinal report of subjective cognitive complaints. Alzheimers Dement (N Y). 2018;4:444-449. doi: 10.1016/j.trci.2018.08.005
25. Insel PS, Hansson O, Mackin RS, Weiner M, Mattsson N; Alzheimer’s Disease Neuroimaging Initiative. Amyloid pathology in the progression to mild cognitive impairment. Neurobiol Aging. 2018;64:76-84. doi: 10.1016/j.neurobiolaging.2017.12.018
26. Sperling RA, Donohue MC, Raman R, et al; A4 Study Team. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77(6):1-11. doi: 10.1001/jamaneurol.2020.0387
27. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3(1):320-332. doi: 10.1159/000354370
28. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252-265. doi: 10.1111/j.1600-0447.2008.01326.x.
29. Foster NL, Bondi MW, Das R, et al. Quality improvement in neurology: mild cognitive impairment quality measurement set. Neurology. 2019;93(16):705-713. doi: 10.1212/WNL.0000000000008259
30. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for cognitive impairment in older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(8):757-763. doi: 10.1001/jama.2020.0435
31. Fink HA, Hemmy LS, Linskens EJ, et al. Diagnosis and Treatment of Clinical Alzheimer’s-Type Dementia: A Systematic Review. Agency for Healthcare Research and Quality (US); 2020.
32. Hemmy LS, Linskens EJ, Silverman PC, et al. Brief cognitive tests for distinguishing clinical Alzheimer-type dementia from mild cognitive impairment or normal cognition in older adults with suspected cognitive impairment. Ann Intern Med. 2020;172(10):678-687. doi: 10.7326/M19-3889
33. Johnson KA, Minoshima S, Bohnen NI, et al; Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9(1):e-1-16. doi: 10.1016/j.jalz.2013.01.002
34. Alzheimer’s Association. For healthcare professionals: frequently asked questions about beta-amyloid imaging. Published 2020. Accessed May 11, 2020. alz.org/media/Documents/health-care-pros-faqs-beta-amyloid-imaging.pdf
35. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286-1294. doi: 10.1001/jama.2019.2000
36. Eli Lilly and Company. Medicines in development. Published April 20, 2020. Accessed May 9, 2020. lilly.com/discovery/clinical-development-pipeline
37. Fleisher AS, Pontecorvo MJ, Devous MD Sr, et al; A16 Study Investigators. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. Published online April 27, 2020. doi: 10.1001/jamaneurol.2020.0528.
38. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26(3):379-386. doi: 10.1038/s41591-020-0755-1
39. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422-433. doi: 10.1016/S1474-4422(20)30071-5
40. Sabbagh MN, Boada M, Borson S, et al. Early detection of mild cognitive impairment (MCI) in primary care. J Prev Alz Dis. 2020;3(7):165-170. doi: 10.14283/jpad.2020.21
41. Wang H, Li T, Barbarino P, et al. Dementia care during COVID-19. Lancet. 2020;395(10231):1190-1191. doi: 10.1016/S0140-6736(20)30755-8
42. Brown EE, Kumar S, Rajji TK, Pollock BG, Mulsant BH. Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry. 2020;28(7):712-721. doi: 10.1016/j.jagp.2020.04.010
43. Yourish K, Lai R, Ivory D, Smith M. One-third of all U.S. coronavirus deaths are nursing home residents or workers. New York Times. Updated May 11, 2020. Accessed July 21, 2020. nytimes.com/interactive/2020/05/09/us/coronavirus-cases-nursing-homes-us.html
44. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205
45. Coupland CA, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi: 10.1001/jamainternmed.2019.0677
46. Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315. doi: 10.1136/bmj.k1315
47. Scott IA, Anderson K, Freeman CR, Stowasser DA. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201(7):390-392. doi: 10.5694/mja14.00146
48. 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. doi: 10.1111/jgs.15767
49. El-Saifi N, Moyle W, Jones C, Tuffaha H. Medication adherence in older patients with dementia: a systematic literature review. J Pharm Pract. 2018;31(3):322-334. doi: 10.1177/0897190017710524
50. Nguyen TA, Gilmartin-Thomas J, Tan EC, et al. The impact of pharmacist interventions on quality use of medicines, quality of life, and health outcomes in people with dementia and/or cognitive impairment: a systematic review. J Alzheimers Dis. 2019;71(1):83-96. doi: 10.3233/JAD-190162
51. Moga DC, Beech BF, Abner EL, et al. INtervention for Cognitive Reserve Enhancement in delaying the onset of Alzheimer’s Symptomatic Expression (INCREASE), a randomized controlled trial: rationale, study design, and protocol. Trials. 2019;20(1):806. doi: 10.1186/s13063-019-3993-0
52. Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for aggressive and agitated behaviors in dementia: a systematic review and network meta-analysis. Ann Intern Med. 2019;171(9):633-642. doi: 10.7326/M19-0993
53. Drugs for cognitive loss and dementia. Med Lett Drugs Ther. 2017;59(1530):155-161.
54. Courtney C, Farrell D, Gray R, et al; AD2000 Collaborative Group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004;363(9427):2105-2115. doi: 10.1016/S0140-6736(04)16499-4
55. Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2018;6(6):CD001190. doi: 10.1002/14651858.CD001190.pub3
56. Rockwood K, Fay S, Gorman M, Carver D, Graham JE. The clinical meaningfulness of ADAS-Cog changes in Alzheimer’s disease patients treated with donepezil in an open-label trial. BMC Neurol. 2007;7:26. doi: 10.1186/1471-2377-7-26
57. McShane R, Westby MJ, Roberts E, et al. Memantine for dementia. Cochrane Database Syst Rev. 2019;3(3):CD003154. doi: 10.1002/14651858.CD003154.pub6
58. Choosing Wisely. American Geriatrics Society. Ten things clinicians and patients should question. Updated April 23, 2015. Accessed May 9, 2020. choosingwisely.org/wp-content/uploads/2015/02/AGS-Choosing-Wisely-List.pdf
59. Niznik JD, Zhao X, He M, et al. Impact of deprescribing AChEIs on aggressive behaviors and antipsychotic prescribing. Alzheimers Dement. 2020;16(4):630-640. doi: 10.1002/alz.12054
60. Niznik JD, Zhao X, He M, et al. Risk for health events after deprescribing acetylcholinesterase inhibitors in nursing home residents with severe dementia. J Am Geriatr Soc. 2020;68(4):699-707. doi: 10.1111/jgs.16241
61. Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808-813. doi: 10.1001/archinte.165.7.808
62. Green AR, Segal J, Boyd CM, Huang J, Roth DL. Patterns of potentially inappropriate bladder antimuscarinic use in people with dementia: a retrospective cohort study. Drugs Real World Outcomes. 2020;7(2):151-159. doi: 10.1007/s40801-020-00181-z
63. Bachurin SO, Bovina EV, Ustyugov AA. Drugs in clinical trials for Alzheimer’s disease: the major trends. Med Res Rev. 2017;37(5):1186-1225. doi: 10.1002/med.21434
64. Howard R, Zubko O, Bradley R, et al; Minocycline in Alzheimer Disease Efficacy (MADE) Trialist Group. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2019;77(2):164-174. doi: 10.1001/jamaneurol.2019.3762
65. Ryan J, Storey E, Murray AM, et al; ASPREE Investigator Group. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 2020;95(3):e320-e331. doi: 10.1212/WNL.0000000000009277.
66. DeKosky ST, Williamson JD, Fitzpatrick AL, et al; Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253-2262. doi: 10.1001/jama.2008.683
67. Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378(4):321-330. doi: 10.1056/NEJMoa1705971
68. Eisai and Biogen to discontinue phase III clinical studies of BACE inhibitor elenbecestat in early Alzheimer’s disease. Eisai. News release. Published September 13, 2019. Accessed May 10, 2020. eisai.mediaroom.com/2019-09-13-Eisai-And-Biogen-To-Discontinue-Phase-III-Clinical-Studies-Of-BACE-Inhibitor-Elenbecestat-In-Early-Alzheimers-Disease
69. Wessels AM, Tariot PN, Zimmer JA, et al. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 2019;77(2):199-209. doi: 10.1001/jamaneurol.2019.3988
70. Roche to discontinue phase III CREAD 1 and 2 clinical studies of crenezumab in early Alzheimer’s disease (AD) - other company programmes in AD continue. Roche. News release. Published January 30, 2019. Accessed May 9, 2020. roche.com/dam/jcr:e3181d56-8cac-4db8-a7d4-2f883ee2847c/en/20190130-MR_CREN_EN.pdf
71. Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2018;378(18):1691-1703. doi: 10.1056/NEJMoa1706441
72. Relkin NR, Thomas RG, Rissman RA, et al; Alzheimer’s Disease Cooperative Study. A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology. 2017;88(18):1768-1775. doi: 10.1212/WNL.0000000000003904
73. Lawlor B, Segurado R, Kennelly S, et al; NILVAD Study Group. Nilvadipine in mild to moderate Alzheimer disease: a randomised controlled trial. PLoS Med. 2018;15(9):e1002660. doi: 10.1371/journal.pmed.1002660
74. Frolich L, Atri A, Ballard C, et al. Open-label, multicenter, phase III extension study of idalopirdine as adjunctive to donepezil for the treatment of mild-moderate Alzheimer’s disease. J Alzheimers Dis. 2019;67(1):303-313. doi: 10.3233/JAD-180595
75. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y). 2019;5:272-293. doi: 10.1016/j.trci.2019.05.008
76. Vandenberghe R, Rinne JO, Boada M, et al; Bapineuzumab 3000 and 3001 Clinical Study Investigators. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther. 2016;8(1):18. doi: 10.1186/s13195-016-0189-7
77. Doody RS, Raman R, Farlow M, et al; Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369(4):341-350. doi: 10.1056/NEJMoa1210951
78. US Food and Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment. Guidance for Industry. Published February 2018. Accessed May 14, 2020. fda.gov/media/110903/download
79. Morant AV, Jagalski V, Vestergaard HT. Labeling of disease-modifying therapies for neurodegenerative disorders. Front Med (Lausanne). 2019;6:223. doi: 10.3389/fmed.2019.00223
80. Alzforum. CTAD lessons for 2020: more phase 2 trials, more diversity. Clinical trials on Alzheimer’s disease 2019. Published January 9, 2020. Accessed May 12, 2020. alzforum.org/news/conference-coverage/ctad-lessons-2020-more-phase-2-trials-more-diversity
81. Cummings J, Feldman HH, Scheltens P. The “rights” of precision drug development for Alzheimer’s disease. Alzheimers Res Ther. 2019;11(1):76. doi: 10.1186/s13195-019-0529-5
82. Cole MA, Seabrook GR. On the horizon—the value and promise of the global pipeline of Alzheimer’s disease therapeutics. Alzheimers Dement (N Y). 2020;6(1):e12009. doi: 10.1002/trc2.12009
83. Alzforum. Aducanumab. Updated July 17, 2020. Accessed July 21, 2020. alzforum.org/
therapeutics/aducanumab
84. Schneider L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 2020;19(2):111-112. doi: 10.1016/S1474-4422(19)30480-6
85. Biogen. Aducanumab update. Updated October 28, 2019. Accessed May 12, 2020. investors.biogen.com/static-files/5a31a1e3-4fbb-4165-921a-f0ccb1d64b65
86. Haeberlein SB, von Hehn C, Tian Y, et al. EMERGE and ENGAGE topline results: two phase 3 studies to evaluate aducanumab in patients with early Alzheimer’s disease. CTAD 2019. December 19, 2019. Accessed May 12, 2020. investors.biogen.com/static-files/ddd45672-9c7e-4c99-8a06-3b557697c06f
87. Alzforum. “Reports of My Death Are Greatly Exaggerated.” Signed, Aducanumab. Published October 24, 2019. Accessed May 12, 2020. alzforum.org/news/research-news/reports-my-death-are-greatly-exaggerated-signed-aducanumab
88. Biogen. Biogen reports Q1 2020 revenues of $3.5 billion. Published April 22, 2020. Accessed May 12, 2020. investors.biogen.com/static-files/60eda571-f103-42ee-8b03-5c64571dfc13
89. Biogen. Biogen completes submission of Biologics License Application to FDA for aducanumab as a treatment for Alzheimer’s disease. Published July 8, 2020. Accessed July 15, 2020. investors.biogen.com/news-releases/news-release-details/biogen-completes-submission-biologics-license-application-fda
90. A Study to Confirm Safety and Efficacy of BAN2401 in Participants With Early Alzheimer’s Disease (Clarity AD). Updated March 16, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT03887455
91. Alzheimer’s Clinical Trials Consortium and Eisai. Alzheimer’s Clinical Trials Consortium selects elenbecestat and BAN2401 for upcoming clinical studies on prevention of Alzheimer’s disease. Published May 10, 2019. Accessed May 9, 2020. eisai.com/news/2019/pdf/enews201932pdf.pdf?fbclid=IwAR2Ou-nfoUw19WxiSIQJv3KR5tgfohjNyPA97bkXCMPEX51enLe1KyLV-6s
92. Alzheimer’s Clinical Trials Consortium. AHEAD 3-45 Study. 2019. Accessed June 10, 2020. a3a45.org
93. Roche provides topline results from investigator-led phase II/III trial with gantenerumab in rare inherited form of Alzheimer’s disease. Roche. News release. February 10, 2020. Accessed May 9, 2020. roche.com/media/releases/med-cor-2020-02-10.htm
94. Gauthier S, Aisen PS, Cummings J, et al; EU/US CTAD Task Force. Non-amyloid approaches to disease dodification for Alzheimer’s disease: an EU/US CTAD Task Force report. J Prev Alzheimers Dis. doi: 10.14283/jpad.2020.182020:1-6
95. Wilcock GK, Gauthier S, Frisoni GB, et al. Potential of low dose leuco-methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer’s disease: cohort analysis as modified primary outcome in a phase III clinical trial. J Alzheimers Dis. 2018;61(1):435-457. doi: 10.3233/JAD-170560
96. Safety and Efficacy of TRx0237 in Subjects With Alzheimer’s Disease Followed by Open-Label Treatment. Updated July 15, 2020. Accessed July 21, 2020. clinicaltrials.gov/ct2/show/NCT03446001
97. Safety and Efficacy Study of ALZT-OP1 in Subjects With Evidence of Early Alzheimer’s Disease (COGNITE). Updated May 1, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT02547818
98. Detke M, Lynch C, Holsinger LJ, et al. Poster P4-663: Initiation of the phase 2/3 GAIN trial of COR388, a novel bacterial virulence factor inhibitor for the treatment of Alzheimer’s disease based on phase 1 a/b safety, PK, biomarker, and efficacy data. Alzheimers Dement. 2019;15(7 suppl):P1585.
99. Cortexyme provides regulatory update for COR388 development program in Alzheimer’s disease. BioSpace. Published February 13, 2020. Accessed May 6, 2020. biospace.com/article/cortexyme-provides-regulatory-update-for-cor388-development-program-in-alzheimer-s-disease/
100. AB Science. Interim results of masitinib study in Alzheimer’s disease: positive trend of efficacy in one of the doses tested. Published June 26, 2019. Accessed May 12, 2020. ab-science.com/images/_pdf/CP_AD_Interim_VEng_VF.pdf
101. Masitinib in Patients With Mild to Moderate Alzheimer’s Disease. Updated April 7, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT01872598
102. Study of AGB101 in Mild Cognitive Impairment Due to Alzheimer’s Disease (HOPE4MCI). Updated April 3, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT03486938
103. Hampel H, Williams C, Etcheto A, et al. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: analysis of the blarcamesine (ANAVEX2-73) phase 2a clinical study. Alzheimers Dement (N Y). 2020;6(1):e12013. doi: 10.1002/trc2.12013
104. OLE for Patients Enrolled in Phase 2b/3 Study ANAVEX2-73-AD-004 (ATTENTION-AD). Updated March 19, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT04314934
105. Vandenberghe R, Riviere ME, Caputo A, et al. Active Aβ immunotherapy CAD106 in Alzheimer’s disease: a phase 2b study. Alzheimers Dement (N Y). 2017;3(1):10-22. doi: 10.1016/j.trci.2016.12.003
106. Lopez Lopez C, Tariot PN, Caputo A, et al. The Alzheimer’s Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimers Dement (N Y). 2019;5:216-227. doi: 10.1016/j.trci.2019.02.005
107. A Study of CAD106 and CNP520 Versus Placebo in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s Disease (Generation S1). Updated June 18, 2020. Accessed July 21, 2020. clinicaltrials.gov/ct2/show/NCT02565511
108. Brain Amyloid and Vascular Effects of Eicosapentaenoic Acid (BRAVE-EPA). Updated April 15, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT02719327
109. Boada M, López O, Núñez L, et al. Plasma exchange for Alzheimer’s disease Management by Albumin Replacement (AMBAR) trial: study design and progress. Alzheimers Dement (N Y). 2019;5:61-69. doi: 10.1016/j.trci.2019.01.001
110. Grifols presents its latest Alzheimer’s clinical trial data. Grifols. News release. Published December 6, 2019. Accessed May 12, 2020. intranet.grifols.com/documents/38081080/1022159504/np-20191206-en.pdf/a779e23c-2c8a-4d5a-915e-f3536f6e5b6e
111. Imbimbo BP, Ippati S, Ceravolo F, Watling M. Perspective: is therapeutic plasma exchange
a viable option for treating Alzheimer’s disease? Alzheimers Dement (N Y). 2020;6(1):e12004. doi: 10.1002/trc2.12004
112. Study of BHV-4157 in Alzheimer’s Disease (T2 Protect AD). Updated February 17, 2020. Accessed May 12, 2020. clinicaltrials.gov/ct2/show/NCT03605667
113. Carlisle BG. Clinical trials stopped by Covid-19. Published May 5, 2020. Accessed May 9, 2020. covid19.bgcarlisle.com/published/full-report-2020-05-07.html
114. Alzforum. Coronavirus takes its toll on Alzheimer’s clinical studies. Published March 27, 2020. Accessed May 9, 2020. alzforum.org/news/community-news/coronavirus-takes-its-toll-alzheimers-clinical-studies
115. Langa KM, Burke JF. Preclinical Alzheimer disease—early diagnosis or overdiagnosis? JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2629. doi: 10.1001/jamainternmed.2019.2629
