- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Continuous Glucose Monitoring Overview: Features and Evidence
To claim CE credit for this activity, please visit https://www.pharmacytimes.org/courses/analyzing-the-value-of-continuous-glucose-monitoring-in-diabetes-care-and-overcoming-barriers-through-expanded-pharmacy-access
Introduction
Diabetes is a complicated, chronic illness that requires frequent and consistent care.1 The prevalence of diabetes has reached global pandemic levels, and is a major cause of morbidity, mortality, and significant resource use worldwide.1,2 Traditionally, blood glucose monitoring (BGM) has been the gold standard for persons with diabetes to self-monitor their blood glucose levels. There is a wide variation in the clinical recommendations and personal agreements between patients and their healthcare providers (HCPs) concerning BGM frequency. However, more frequent testing is associated with more accurate glycemic control.3 The variation in testing frequency is also complicated by the lack of adherence to BGM testing agreed to, which will help delay or prevent diabetes-related complications.4,5 There can also be significant costs associated with obtaining testing supplies, and fingersticks are painful and burdensome to patients, as some require upwards of 4 to 10 per day.1 If the cost of a single test strip is $1.16, a patient performing BGM twice daily would spend $2.32 per day or $69.60 monthly in addition to incur-ring the initial charge of the actual meter. Annual costs for BGM range from $1000 to $3000.6 A continuous glucose monitor (CGM) will cost between $160 and $500 per month but will allow patients to acquire between 2800 and 20,160 interstitial glucose readings over the 10- to 14-day sensor life.7 Thus, CGM can be performed for less than $0.007 per test.
Epidemiology
The Centers for Disease Control and Prevention estimated that in 2019, 11.3% of the US population (37.3 million people) had diabetes, including 8.5 million Americans who were undiagnosed.8 By 2050, 1 in 3 Americans will be diagnosed with diabetes during their life-time.9 Significant racial and ethnic disparities still exist, with the prevalence of diagnosed diabetes in 2019 having been the highest in American Indian/Alaskan Native (14.5%), non-Hispanic Black (12.1%), and Hispanic (11.8%) individuals compared with non-Hispanic Asian (9.5%) and non-Hispanic White (7.4%) individuals.10 An estimated 96 million adults 18 years or older had prediabetes in 2019, and 48.8% of that population is over the age of 65.8
Diabetes-Related Complications
Risk Factors
Because diabetes is a complex, chronic illness, multiple strategies are necessary to decrease risks in addition to glycemic control.1 Approximately 90% of all persons with diabetes in the United States have insulin resistance (type 2 diabetes [T2D]) in which modifiable risk factors exist, whereas the remaining 10% have autoimmune or type 1 diabetes (T1D).1,11 For example, smoking, weight, physical inactivity, high blood pressure, high cholesterol, and A1C can be modified to delay or prevent the progression of diabetes-related complications.1,11
Comorbidities and Complications
Comorbidities and complications of diabetes include cardiovascular (CV) disease, chronic kidney disease (CKD), neuropathy, dental caries, diabetic foot problems, mental health problems, hearing loss, and macular degeneration, a severe form of diabetic retinopathy leading to vision loss.12 Other acute issues such as hypoglycemia, diabetic ketoacidosis, hyperglycemic hyperosmolar state, and hyperglycemic diabetic comas can also occur. Fortunately, with proper care and preventive measures, most complications can be delayed or avoided, allowing affected patients to live longer and healthier lives.12,13
Macrovascular Complications
Atherosclerotic CV disease (ASCVD), including coronary heart disease, cerebrovascular disease, and peripheral arterial disease, is the leading cause of morbidity and mortality in persons with diabetes. There is significant evidence showing the benefits of controlling CV risk factors to slow the onset and/or progression of ASCVD among persons with diabetes. CV risk factors should always be assessed in patients with diabetes (at minimum annually), including duration of diabetes, obesity or overweight, hypertension, dyslipidemia, smoking, family history of ASCVD, CKD, and albu-minuria (a marker of inflammation).14 A multifactorial approach (glycemic/hypertension/dyslipidemia management and use of agents with CV and renal benefits) where risk factors are addressed simultaneously is strongly recommended to improve CV outcomes among persons with diabetes.1
Microvascular Complications
Diabetic kidney disease is a microvascular complication of T1D and T2D occurring in 30% to 40% of patients and is a major contributor to mortality in diabetes.15 CV morbidity and mortality among people with diabetic kidney disease is 20 to 40 times higher compared with persons with diabetes without nephropathy.16 Diabetic retinopathy is another frequent complication present in almost 20% of patients at the time of their diabetes diagnosis, with another 40% to 45% of persons with diabetes developing it over their disease course.15
Glucose (or glycemic) variability (GV) has a renewed focus on its potential role in the development of diabetes complications.17 There is no question that poor glycemic control, regardless of the type of diabetes, can lead to severe glycemic events requiring hospitaliza-tion.18 Because severe hyperglycemia/hypoglycemia and diabetic ketoacidosis can result in diabetic coma and death, it is essential that blood glucose is monitored frequently, as these events are preventable.18 Patients with plasma glucose levels greater than 180 mg/dL at the time of a stroke or myocardial infarction are at a higher risk of mortality. This is because neurons, nephrons, retinal cells, and endothelial cells are inefficient interstitial transporters of glucose, so as glucose levels rise above 180 mg/dL within these “at-risk cells,” a cascade of events occurs, leading to acute compli-cations and cell death.19
Limitations of Blood Glucose Monitoring and A1C for Glycemic Monitoring
Limitations of Blood Glucose Monitoring
BGM has historically been the standard method by which persons with diabetes monitor their glucose levels on a day-to-day basis. BGM has allowed for improved patient outcomes and patient quality of life (QOL) by allowing for improved recognition of hypogly-cemia and hyperglycemia and motivating patients to continue to improve.20 Despite being the current standard of care for glucose monitoring, BGM is not without limitations, both from human error and machine error. Human error can consist of improper use of the glucose meter or strips, improper device setup, infrequent testing or nonadherence, or incomplete patient education about using the device properly. Machine error can consist of device errors, accuracy, ease of use, or faulty test strips and meters. There can also be physiologic errors that cause inaccurate glucose readings, such as variable hematocrit levels. Accurate BGM is essential for insulin-requiring patients to be able to provide themselves with appropriate prandial insulian doses.20,21
Limitations of A1C for Monitoring
Glycosylated hemoglobin A1C, which estimates the average blood glucose over the preceding 8 to 12 weeks, was discussed as a potential diagnostic tool at an International Expert Committee in 2008 and was later accepted as a diagnostic tool by the American Diabetes Association (ADA).22 Since then, the ADA has implemented A1C as a monitoring tool standard of care, with goal A1C recommenda-tions of less than 7% to 8% based on specific patient parameters.23 Although the A1C’s interindividual variability is lower than blood glucose measurements, there are limitations that can cause falsely low or high A1C measurements.24 Some conditions that could falsely lower an A1C measurement include CKD with or without hemodialysis, significant blood loss requiring blood transfusion, pregnancy, or patients on erythropoietin therapy. Essentially, any condition that can increase the rate of erythrocyte creation or increase the rate of erythrocyte removal can lead to a falsely low A1C. Patients with conditions that increase the lifespan of eryth-rocytes, such as anemia or asplenia, can have falsely elevated A1C measurements.25,26 Furthermore, A1C does not measure GV or rapid changes in daily glucose controls such as excursions of hypogly-cemia and hyperglycemia.23,24
Continuous Glucose Monitoring and Diabetes Care
Evolution of Diabetes Management and Continuous Glucose Monitoring Devices
Before discovering insulin in 1921, managing persons with diabetes required severe caloric restriction and avoidance of ketoacidosis.27 The diagnosis of diabetes prior to 1921 was akin to a death sentence, with most patients succumbing to the disease within 1 year. In 2022, despite having access to many different therapeutic glucose-lowering therapies, only 47% of patients are achieving their recommended A1C target of less than or equal to 7%.28 Patients experience the burden of spending several hours each day attempting to eat prop-erly, exercise, take multiple medications, perform BGM, and attend their clinic and specialty appointments while ultimately failing to achieve their recommended glycemic targets suggested by their treat-ment team. Perhaps patients can reduce their diabetes distress by being provided with the tools needed to achieve more time within a glycemic target range (70-180 mg/dL) while minimizing their risk of treatment-emergent hypoglycemia. Patients seek medical consultation in order to minimize their risk of developing short- and long-term diabetes-related complications. HCPs are obligated to provide patients with the medications, lifestyle interventions, and the tools they need to live life to the fullest. The idea of an implantable glucose sensor to assist patients has been around for over 40 years.29 This concept came to fruition in 1999 to 2000 with the first transdermal glucose sensors available on the market.30 However, these sensors’ utility was limited due to significant drift in sensitivity over the initial US Food and Drug Administration (FDA)-approved 3-day implantation period.31,32 The in vivo sensor output drift was unexpected and, as a result, there was less enthusiasm concerning CGM in the early days of the technology.33,34 However, as the technology improved, there was increased uptake, with most CGM use taking place within endocrinology and diabetes specialty practices.35 Since then, CGM devices have undergone significant technological advancements improving accuracy, smaller, less invasive devices, extended sensor life, alarms, and other features that have improved patient satisfac-tion and adherence to CGM use and medications. The majority of these benefits have been seen in persons with T1D, but there are also data for T2D in people being treated with intensive insulin therapy.36-39 In addition, CGM technology improvements have also led to advancements in integrating with continuous subcutaneous insulin infusion (CSII) pumps and automated insulin delivery systems (“closed-loop systems”).40
Continuous Glucose Monitoring Devices
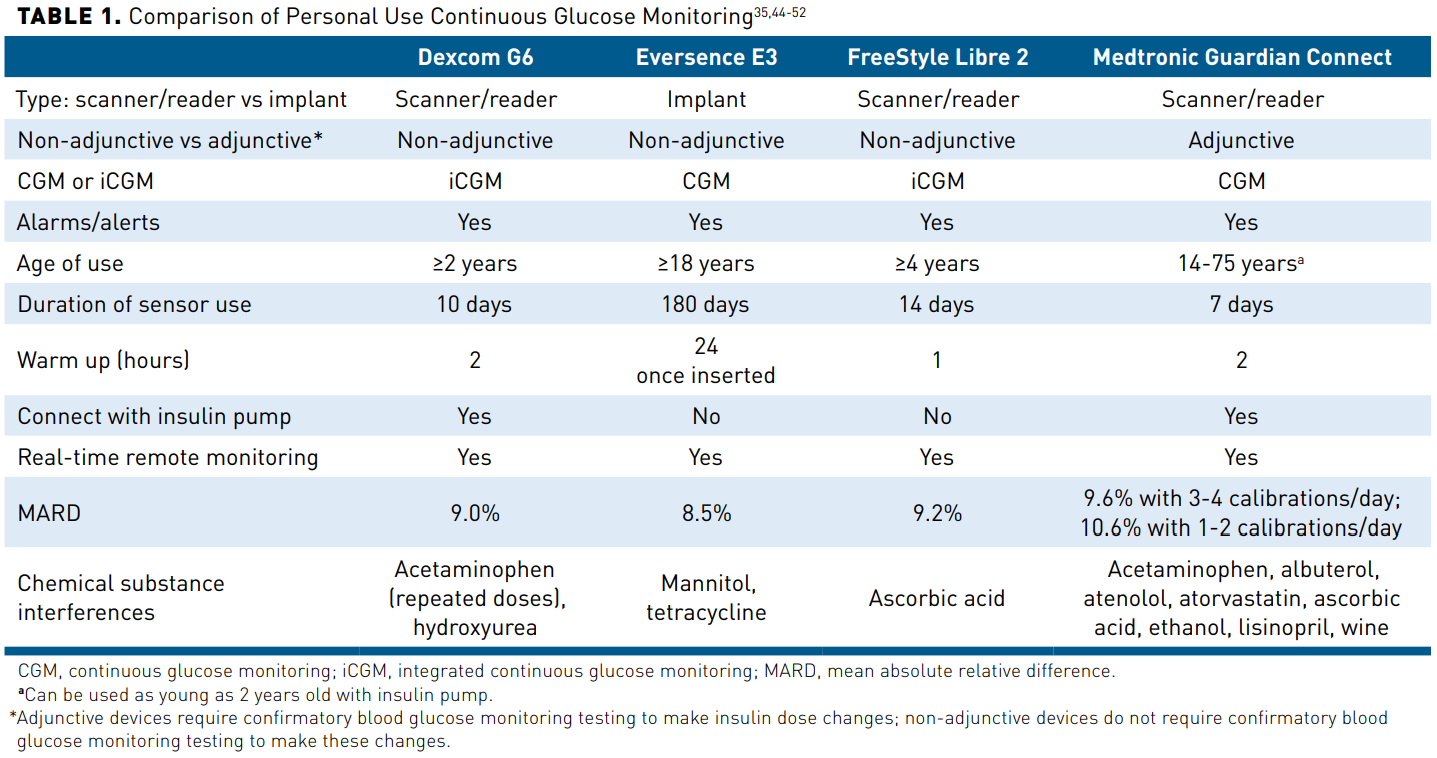
There are 2 types of CGM devices: personal and professional.1 A personal CGM device is owned by the patient and is intended for frequent or continued use, whereas a professional CGM device, which is owned by the provider’s office, not the patient, is placed on the patient in the clinic and is worn for a finite period of time (usually 7-14 days).1 Personal CGM devices, the focus of this activity, are further strati-fied as real-time CGM (rtCGM), which continuously measures and stores glucose levels without prompting, or intermittently scanned (also known as “flash”) CGM (isCGM), which also measures glucose continuously, but requires scanning for storage of values.1 Personal CGM devices are approved for either adjunctive or non-adjunctive use. Adjunctive devices require confirmatory BGM testing to make insulin dose changes, whereas non-adjunctive devices do not require confirmatory BGM testing to make these changes.41 Some personal CGM devices have also been given the designation of integrated CGM (iCGM), which is “designed to reliably and securely transmit glucose measurement data to digitally connected devices, including automated insulin dosing systems, and is intended to be used alone or in conjunction with these digitally connected medical devices for the purpose of managing a disease or condition related to glycemic control.”42 To meet this higher standard set by the FDA, iCGM devices must meet a more stringent accuracy requirement: glucose readings within 15% of the laboratory glucose value when glucose is higher than 70 mg/dL and within 15 mg/dL when glucose is lower than 70 mg/dL. These thresholds must be met at least 70% of the time when glucose levels are between 70 and 180 mg/dL; more than 80% of the time when glucose is higher than 180 mg/dL; and more than 85% of the time when glucose is lower than 70 mg/dL.43 Currently, Dexcom G6 and FreeStyle Libre 2 are the only devices that meet this iCGM criteria.42 Historically, the major differences between the CGM devices were whether they could provide real-time alerts and alarms regarding glucose levels and the person’s target goals.41 Presently, all personal CGM devices have alert/alarm functions and can help the person with diabetes manage acute glycemic events based on high and low thresholds and provide immediate feedback regarding their lifestyle or therapy decisions (eg, diet, exercise, and medica-tion nonadherence). Regulations also require all CGM devices to have an automatic low alarm for blood glucose at 55 mg/dL that cannot be silenced by the user of the device.41 See Table 135,44-52 for a comparison of currently available personal CGM devices. The FreeStyle Libre 3 was cleared for use by the FDA in May 2022. Both the FreeStyle Libre 2 and the FreeStyle Libre 3 have a 14-day sensor life and customizable glucose alarms, which allow the user time to react to mild hypoglycemia rather than requiring assistance from a third party. The FreeStyle Libre 3 is an rtCGM, whereas the FreeStyle Libre 2 is an isCGM.46,53 Additionally, the FreeStyle Libre 3 has the lowest mean absolute relative difference (MARD), an accu-racy standard used for CGM, of all available CGM devices at 7.9%.54
Continuous Glucose Monitoring as the New Standard of Care
The updated 2022 ADA Standards of Care have expanded their recommendations to include CGM devices as the preferred glucose monitoring device for adults on insulin.1 Specifically, CGM is recom-mended in adults on multiple daily injections (MDI), those solely on basal insulin, or CSII.1 It is also recommended in youth (T1D/T2D) who are on MDI or CSII and as an adjunct to BGM in pregnancy. The ADA recommends a 14-day CGM assessment of time in range (TIR) and glucose management indicator (GMI) be used to accu-rately assess a patient’s risk of hypoglycemia and hyperglycemia. TIR allows for a more real-time assessment of a patient’s risk when compared with A1C.1 The 2021 American Association of Clinical Endocrinology (AACE) guidelines suggest that all persons with diabetes, regard-less of insulin dependency, may benefit from the use of CGM (see Box55). Furthermore, the AACE recommends using an rtCGM over an isCGM for patients on insulin or at higher risk of hypoglycemia. A 14-day CGM evaluation timeframe and a TIR goal of greater than 70% remains the same between the 2 organizations. Individualizing care for each person is also recommended by both organizations.1,55

Overview of Continuous Glucose Monitoring Metrics
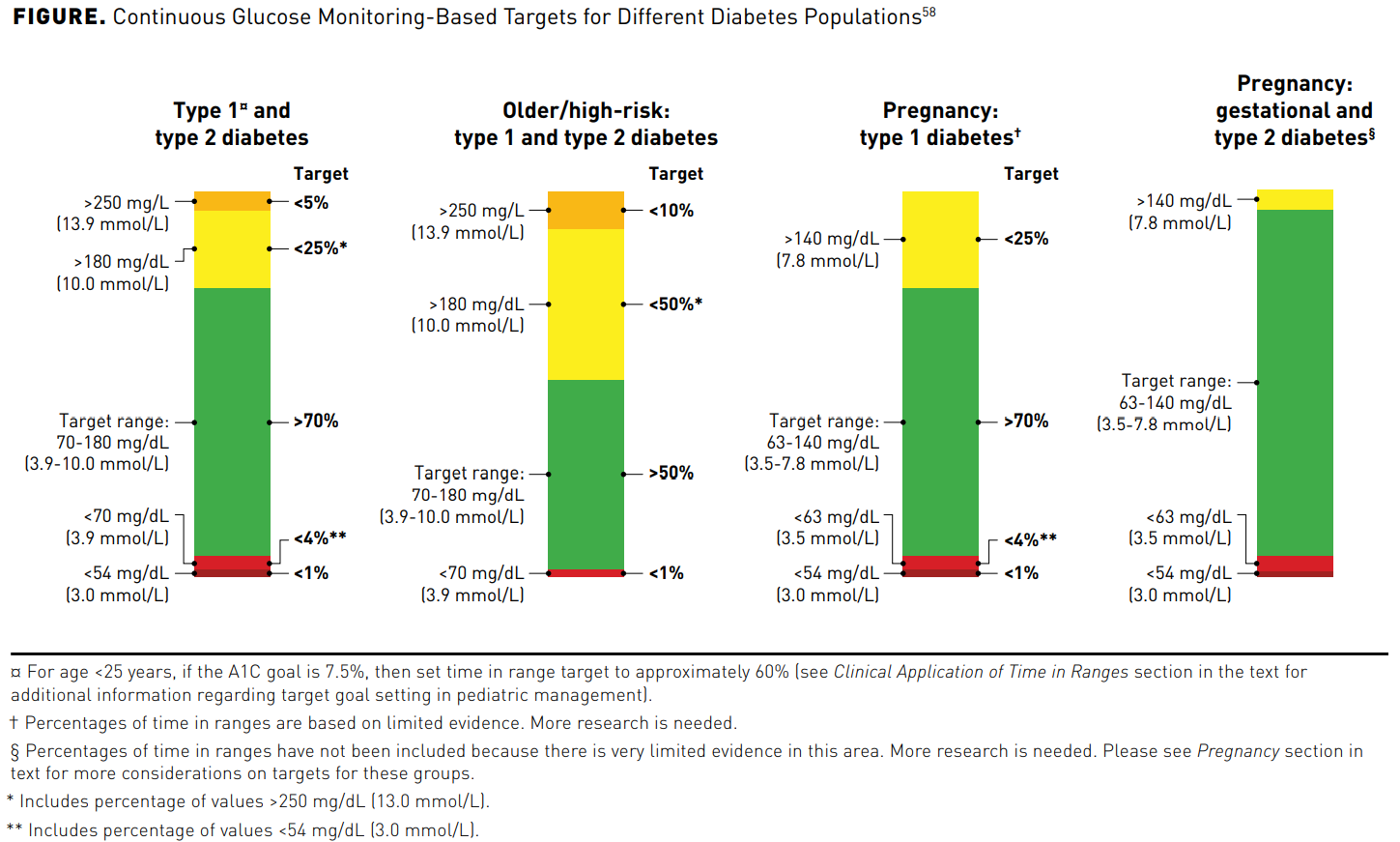
CGM measures glucose in the interstitial fluid every 1 to 5 minutes.56Key metrics on the ambulatory glucose profile are defined in Table 257-59 and their targets are illustrated in the Figure.58 A more detailed discussion of the ambulatory glucose profile and inter-pretation of the metrics is in part 2 of this supplement. A recent study in persons with T1D concluded that 14 days of CGM data correlates well with glucose metrics, including mean glucose, TIR, and hyperglycemia, for a 3-month period.60 Having at least 70% (10 days) of CGM data within that time period adds reliability to these data.59 A real-world evidence study of over 50,000 readers demonstrated that more frequent CGM scanning was associated with improved glycemic markers such as A1C, increased TIR, and reduced hypoglycemia.61
Average Glucose and Glucose Management Indicator
The average glucose is the mean reported glucose value (mg/dL) that is calculated from the data, whereas the GMI is an estimated representation of glycemic control based on a mathematical calcula-tion of thousands of data points attained over the life of the sensor and presented in a format similar to that of A1C (%).57,59 While this estimated A1C (eA1C) can be helpful, it can also confuse or frus-trate people if it differs from subsequent laboratory-measured A1C. Experts have also opined that the term eA1C implies a more direct relationship with the measured A1C than found in reality. As a result of these points of contention, the FDA Center for Devices and Radiological Health determined that changing the nomenclature would be prudent and, with input from various stakeholders (eg, endocrinologists, certified diabetes care and education specialists, and patient advocates), changed the term eA1C to GMI.62 Regardless, GMI may differ from a laboratory-measured A1C because the red blood cell lifespan and how glucose binds to hemoglobin in red blood cells differs among patients.62
Glucose Variability
GV shows the degree of glucose fluctuations (amplitude) and frequency of those variations compared with the mean glucose.62 Maintaining GV less than 36% of the time is currently a therapeutic goal in diabetes management.63,64 Its importance relates to its predic-tive value. Scientific evidence proposes that GV may be associated with increased risk of diabetic microvascular and macrovascular complications, which likely occur from oxidative stress, as well as chronic, low-grade inflammation, endothelial dysfunction, glyca-tion, impaired angiogenesis, platelet activation, and renal fibrosis. In addition, a few studies indicate that high GV may be linked to mortality in persons with T2D.63 A high GV value might indicate the patient is receiving too much insulin; other signals include basal insulin doses of more than 0.5 international units/kg, large differ-ences in bedtime and morning glucose levels, large differences in glucose levels before and after meals, and hypoglycemia.1,64
Times in Range
TIR is a measurement presented as the percent the patient’s blood glucose is in range, defined as 70 to 180 mg/dL for persons with T1D and T2D and 63 to 140 mg/dL for pregnant women (see Table 135,44-52 and Figure58). Achieving and maintaining TIR is important for all persons with diabetes. Ideally, the overarching goal for most persons with diabetes is to have TIR at least 70% of the time, while avoiding unaccept-able glycemic peaks and troughs (see Figure58). The Advanced Technologies & Treatments for Diabetes meeting selected this TIR target because it approximates an A1C of less than 7%, the A1C value that significantly reduces the risk of diabetes complications.24 For older or high-risk persons with diabetes, the goal is greater than 50% TIR (see Figure58). If we are targeting tighter control based on charac-teristics such as good health and a long-life expectancy, we may consider a target TIR of 80%.58 When 70% of the glucose readings are within range, one can predict the A1C will be close to 7%. Improving a patient’s TIR from using CGM can reduce the risk of macrovascular and microvascular complications.65,66 Time below range (TBR) is defined as less than 70 mg/dL. For most patients the TBR goal is less than or equal to 4%; however, older patients or high-risk patients should lessen their TBR to less than or equal to 1% (see Figure58). Hypoglycemic events over time contribute to complications and cause physical and psycho-social morbidity, and sometimes mortality. Asymptomatic hypoglycemia further compli-cates care and well-being because it may lead to severe hypoglycemia. Population data have shown that up to 40% of persons with T1D experience up to 3 severe hypoglycemic episodes each year.67 Moreover, nocturnal hypoglycemia is especially concerning and responsible for about half of severe hypoglycemic events.68 In persons with T2D, the risk increases with the use of insulin and secretagogues, lack of glycemic control, kidney disease, and impaired cognition and function.69 Severe hypoglycemia is associated with increased morbidity and mortality.70,71
Evidence Supporting the Use of Continuous Glucose Monitoring
Accuracy of Continuous Glucose Monitoring The accuracy of CGM cannot be directly compared with BGM due to the differing method used to measure glucose. CGM measures the glucose within the interstitial fluid while, as the name suggests, BGM measures blood glucose.72 As mentioned previously, MARD is an accuracy standard used for CGM that is relative to the specific device and is influenced by how the CGM device was initially studied. As a result, this makes MARD difficult to use to compare different CGM products. Patient-specific accuracy relies less on the MARD of the product and more on the consistency of CGM use and TIR.73,74 Depending on the CGM device, CGM takes between 288 and 1440 glucose measurements per day.75 Based on these readings over 15 minutes, a rate of change trend arrow is displayed on the CGM reader, indicating whether glucose levels are increasing or decreasing. Because these data are retrospective, it is essential that people using CGM devices are educated on how to appropriately use trend arrows, but that treatment decisions should always occur based on current CGM data instead of trend arrows.75
Clinical and Quality of Life Outcomes
The clinical evidence for CGM devices for persons with diabetes is mirrored in the ADA and the AACE guidelines. Both recom-mend using over-standard glucometers, if available, depending on specific patient factors.1,55 The clinical efficacy and safety of rtCGM has been shown in numerous studies with persons with T1D and T2D, independent of their method and frequency of administering insulin.76-88 The DIAMOND trials showed rtCGM improved A1C, reduced the time spent in hypoglycemic and hyperglycemic ranges, and reduced moderate to severe hypoglycemia in people using MDI compared with traditional BGM in T1D and T2D.76,79 Similarly, the IN CONTROL trial demonstrated that rtCGM use significantly increased time spent in normoglycemia and decreased severe hypoglycemia in people with impaired hypoglycemia awareness.88 Another trial, HypoDE, found rtCGM compared with BGM in adult persons with T1D treated with MDI, impaired hypoglycemia awareness, and frequent severe/nocturnal hypoglycemia had fewer hypoglycemic events and severe hypoglycemia.89
Among persons with T1D/T2D treated with intensive insulin therapy, isCGM is associated with reduced hypoglycemia, increased TIR, and decreased GV.90,91 A 38% reduction in time spent in hypo-glycemia (<70 mg/dL), increased TIR, and reduced GV was also shown in the IMPACT trial.90 Similarly, a 43% reduction in time spent in hypoglycemia was observed in persons with T2D on inten-sive insulin therapy in the REPLACE trial.91 Significantly decreased hospitalizations for hypoglycemia were also seen among persons using isCGM.92,93The benefits for overall patient QOL are minimal in the litera-ture. The DIAMOND study, published in 2017, investigated the QOL of persons with T1D while using their CGM devices. Participants reported a significant decrease in diabetes distress and a significant increase in hypoglycemic confidence, but no changes in well-being, health status, or hypoglycemic fear were seen between the CGM group and the non-CGM group.86 The recent FUTURE obser-vational study in persons with T1D showed isCGM use resulted in higher treatment satisfaction, less severe hypoglycemia, and less work absenteeism.92 Another recent trial, the Landmark study, is the first prospective, real-world study that examined glycemic data and QOL outcomes in persons with T1D or T2D receiving intensive insulin therapy and who have started on an rtCGM device.94 After 12 weeks, diabetes distress and hypoglycemic concerns decreased significantly, independent of the type of diabetes. While there have been several randomized controlled trials that are associated with improved QOL, more robust evidence is needed to fully elucidate the QOL benefits associated with CGM use. Emerging Evidence for Basal Insulin UsersCGM use in persons with diabetes has traditionally been reserved for those with T1D or T2D on prandial insulin.1 However, evidence is beginning to emerge that supports expanding the current CGM recommendations to include persons with diabetes who are on basal insulin with no prandial insulin. Fokkert et al suggested that 37% of patients with an iCGM device reported increased engage-ment in physical activity because the devices lowered the risk of exercise-induced hypoglycemia.93
Opportunities in Telehealth
Isolation requirements for patients diagnosed with COVID-19 and diabetes created a unique challenge for managing blood glucose in both inpatient and outpatient settings.95 Diabetes is a common comorbidity for COVID-19, and with the use of dexamethasone and other corticosteroids being used for COVID-19 treatment, a safe approach for glucose monitoring was needed.96-98 Shen et al have demonstrated that persons with diabetes infected with COVID-19 have an increased risk of adverse outcomes when glucose levels exceed 160 mg/dL or fall below 70 mg/dL. Additionally, exces-sive GV increases the risk of mortality.99 The AACE guidelines encourage hospitalized patients to continue use of their CGM device as long as they are capable of scanning or managing their sensor devices.53
In addition, advances in digital technologies have improved diabetes care delivery.100 The ability of people to interact remotely with their HCPs can increase access to care, online coaching, and support programs.101-103 CGM devices in particular can automatically transmit glucose data to the patient’s HCP, which can be reviewed prior to a face-to-face or telehealth visit. Furthermore, multiple studies have shown improved clinical outcomes from remote monitoring of CGM data via telemedicine visits.101,104-108
Conclusions
Diabetes is a major health concern in the United States and glob-ally. The prevalence of this disease continues to rise exponentially and contributes significantly to morbidity, mortality, and health-care resource utilization. Historically, BGM was the gold standard for monitoring blood glucose levels at home. However, due to the high cost, burden of testing, and lack of adherence to testing, many patients do not test their blood glucose frequently enough. As a result, increased emphasis has been placed on using CGM technology, which can significantly reduce the risk of treatment-emergent hypoglycemia, improve A1C levels, reduce hospitalizations, reduce diabetes complications, and improve absenteeism. CGM can also improve QOL as patients adapt behavioral interventions and exercise programs more confidently while using CGM devices. All persons with diabetes should be encouraged to adapt CGM into their daily treatment regimen.
Author affiliation: Jeff Unger, MD, FAAFP, FACE, is the Director, Unger Primary Care Concierge Medical Group, Cucamonga, CA.
Funding source: This activity is supported by an educational grant from Abbott Diabetes Care Inc.
Author disclosure: Dr Unger has the following relevant financial relation-ships with commercial interests to disclose: Consultant: Abbott Diabetes Care Inc, Dexcom; Honoraria: Abbott Diabetes Care Inc; Lecture fees: Abbott Diabetes Care Inc, Novo Nordisk
Authorship information: Analysis and interpretation of data; concept and design; critical revision of the manuscript for important intellectual content; drafting of the manuscript; supervision.
Address correspondence to: jungermd@aol.com
Medical writing and editorial support provided by: Brittany Hoffmann-Eubanks, PharmD, MBA; Angel A Rodriguez, PharmD; Jeannette Y. Wick, MBA, RPh, FASB, FASCP
REFERENCES
- American Diabetes Association. Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10-38. doi: 10.2337/cd22-as01
- IDF diabetes atlas 2021. International Diabetes Federation. Accessed June 2, 2022. https://diabetesatlas.org/
- Wright LAC, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypo-glycemia, and other parameters. Diabetes Technol Ther. 2017;19(S2):S16-s26. doi: 10.1089/dia.2017.0029
- Centers for Disease Control and Prevention (CDC). Self-monitoring of blood glucose among adults with diabetes—United States, 1997-2006. MMWR Morb Mortal Wkly Rep. 2007;56(43):1133-1137.
- Vincze G, Barner JC, Lopez D. Factors associated with adherence to self-monitoring of blood glucose among persons with diabetes. Diabetes Educ. 2004;30(1):112-125. doi: 10.1177/014572170403000119
- Jacob D. How much does a glucose monitor cost? MedicineNet. Reviewed August 18, 2021. Accessed June 2, 2022. www.medicinenet.com/how_much_does_a_glucose_monitor_cost/article.htm
- Hoskins M. When you can’t afford a continuous glucose monitor. Healthline. Published May 10, 2021. Accessed June 2, 2022. www.healthline.com/diabetesmine/when-you-cant-afford-a-cgm
- Estimates of diabetes and its burden in the United States. Centers for Disease Control and Prevention. Reviewed January 18, 2022. Accessed June 2, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes preva-lence. Population Health Metrics. 2010;8(29). doi: 10.1186/1478-7954-8-29
- Prevalence of diagnosed diabetes. Centers for Disease Control and Prevention. Updated December 29, 2021. Accessed June 2, 2022. www.cdc.gov/diabetes/data/statistics-report/diagnosed-diabetes.html
- National diabetes statistics report. Centers for Disease Control and Prevention. Accessed June 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Prevent diabetes complications. Centers for Disease Control and Prevention. Reviewed March 9, 2022. Accessed June 2, 2022. www.cdc.gov/diabetes/managing/problems.html
- Cost-effectiveness of diabetes interventions. Centers for Disease Control and Prevention. Reviewed March 7, 2022. Accessed June 2, 2022. www.cdc.gov/chronicdisease/programs-impact/pop/diabetes.htm
- Winter L, Wong LA, Jerums G, et al. Use of readily accessible inflammatory markers to predict dia-betic kidney disease. Front Endocrinol (Lausanne). 2018;9:225. doi: 10.3389/fendo.2018.00225
- Demir S, Nawroth PP, Herzig S, Ustunel BE. Emerging targets in type 2 diabetes and diabetic compli-cations. Adv Sci (Weinh). 2021;8(18):e2100275. doi: 10.1002/advs.202100275
- Ricciardi CA, Gnudi L. Kidney disease in diabetes: from mechanisms to clinical presentation and treatment strategies. Metabolism. 2021;124:154890. doi: 10.1016/j.metabol.2021.154890
- Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 2022;10(1):75-84. doi: 10.1016/s2213-8587(21)00245-x
- Roussel R, Riveline JP, Vicaut E, et al. Important drop in rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study. Diabetes Care. 2021;44(6):1368-1376. doi: 10.2337/dc20-1690
- Unger J. Exploring the association between diabetes and cardiovascular disease. In: Schwartz Z, ed. Diabetes Management in Primary Care. 2nd ed. Lippincott Williams and Wilkins; 2013: 217-259.
- Klonoff DC. Benefits and limitations of self-monitoring of blood glucose. J Diabetes Sci Technol. 2007;1(1):130-132. doi: 10.1177/193229680700100121
- Erbach M, Freckmann G, Hinzmann R, et al. Interferences and limitations in blood glucose self-testing: an overview of the current knowledge. J Diabetes Sci Technol. 2016;10(5):1161-1168. doi: 10.1177/1932296816641433
- Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184-190. doi: 10.2337/dc11-s216
- Draznin B, Aroda VR, Bakris G, et al; American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83-S96. doi: 10.2337/dc22-S006
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. doi: 10.2337/dc17-1600
- Guo W, Zhou Q, Jia Y, Xu J. Increased levels of glycated hemoglobin A1c and iron deficiency anemia: a review. Med Sci Monit. 2019;25:8371-8378. doi: 10.12659/msm.916719
- Shepard JG, Airee A, Dake AW, McFarland MS, Vora A. Limitations of A1c interpretation. South Med J. 2015;108(12):724-729. doi: 10.14423/SMJ.0000000000000381
- Vecchio I, Tornali C, Bragazzi NL, Martini M. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol (Lausanne). 2018;9:613. doi: 10.3389/fendo.2018.00613
- Hirsch IB, Verderese CA. Professional flash continuous glucose monitoring with ambulatory glucose profile reporting to supplement A1C: rationale and practical implementation. Endocr Pract. 2017;23(11):1333-1344. doi: 10.4158/ep171962.Ra
- Hirsch IB, Battelino T, Peters AL, Chamberlain JJ, Aleppo G, Bergenstal RM. Role of continuous glucose monitoring in diabetes treatment. American Diabetes Association, Abbott. Accessed June 2, 2022. https://professional.diabetes.org/sites/professional.diabetes.org/files/media/final_ada-abbott_cgm_compendium_final.pdf
- Didyuk O, Econom N, Guardia A, Livingston K, Klueh U. Continuous glucose monitoring devices: past, present, and future focus on the history and evolution of technological innovation. J Diabetes Sci Technol. 2021;15(3):676-683. doi: 10.1177/1932296819899394
- Gerritsen M, Jansen JA, Kros A, et al. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mater Res. 2001;54(1):69-75. doi: 10.1002/1097-4636(200101)54:1<69::aid-jbm8>3.0.co;2-q
- Gerritsen M, Jansen JA, Lutterman JA. Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth J Med. 1999;54(4):167-179. doi: 10.1016/s0300-2977(99)00006-6
- Gerritsen M. Problems associated with subcutaneously implanted glucose sensors. Diabetes Care. 2000;23(2):143-145. doi: 10.2337/diacare.23.2.143
- Wisniewski N, Klitzman B, Miller B, Reichert WM. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57(4):513-521. doi: 10.1002/1097-4636(20011215)57:4<513::aid-jbm1197>3.0.co;2-e
- Galindo RJ, Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. doi: 10.1016/j.diabres.2020.108502
- Ajjan RA, Jackson N, Thomson SA. Reduction in hba1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: a pilot, multi-centre, randomised controlled trial. Diab Vasc Dis Res. 2019;16(4):385-395. doi: 10.1177/1479164119827456
- Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203-210. doi: 10.1089/dia.2007.0205
- New JP, Ajjan R, Pfeiffer AF, Freckmann G. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32(5):609-617. doi: 10.1111/dme.12713
- Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: a narrative review. J Diabetes Investig. 2018;9(4):713-725. doi: 10.1111/jdi.12807
- Beyond A1C Writing Group. Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care. 2018;41(6):e92-e94. doi: 10.2337/dci18-0010
- Hirsch IB, Miller E. Integrating continuous glucose monitoring into clinical practices and patients’ lives. Diabetes Technol Ther. 2021;23(S3):S72-s80. doi: 10.1089/dia.2021.0233
- Food and Drug Administration. Medical devices; clinical chemistry and clinical toxicology devices; classification of the integrated continuous glucose monitoring system. Federal Register. Published February 18, 2022. Accessed June 2, 2022. www.federalregister.gov/documents/2022/02/18/2022-03504/medical-devices-clinical-chemistry-and-clinical-toxicology-devices-classification-of-the-integrated
- Garg SK, Akturk HK. A new era in continuous glucose monitoring: Food and Drug Administration creates a new category of factory-calibrated nonadjunctive, interoperable class ii medical devices. Diabetes Technol Ther. 2018;20(6):391-394. doi: 10.1089/dia.2018.0142
- Summary of safety and effectiveness data (SSED): Guardian Connect System. FDA. Accessed June 2, 2022. www.accessdata.fda.gov/cdrh_docs/pdf16/P160007b.pdf
- Evaluation of automatic class III designation for Dexcom G6 Continuous Glucose Monitoring System. U.S. Food and Drug Administration. Accessed June 2, 2022. www.accessdata.fda.gov/cdrh_docs/reviews/DEN170088.pdf
- FreeStyle Libre 2 Flash Glucose Monitoring System. U.S. Food and Drug Administration. Accessed June 2, 2022. www.accessdata.fda.gov/cdrh_docs/pdf19/K193371.pdf
- Summary of safety and effectiveness data (SSED): Eversence E3 Continuous Glucose Monitoring System. U.S. Food and Drug Administration. Accessed June 2, 2022. www.accessdata.fda.gov/cdrh_docs/pdf16/P160048S016B.pdf
- Full indications and important safety information FreeStyle Libre 14 day. Abbott Diabetes Care. Published May 2022. Accessed June 2, 2022. www.freestyle.abbott/us-en/safety-information.html
- Basu A, Slama MQ, Nicholson WT, et al. Continuous glucose monitor interference with commonly prescribed medications: a pilot study. J Diabetes Sci Technol. 2017;11(5):936-941. doi: 10.1177/1932296817697329
- Dexcom G6 user guide. Dexcom. Accessed June 2, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/HCP_Website/LBL015752+Rev+003+Artwork%2C+G6+Using+Your+G6+US+WEB.PDF
- Lorenz C, Sandoval W, Mortellaro M. Interference assessment of various endogenous and exogenous substances on the performance of the eversense long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2018;20(5):344-352. doi: 10.1089/dia.2018.0028
- Guardian Connect system user guide. Medtronic. Accessed June 2, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/GuardianT%20Connect%20CGM%20System%20User%20Guide.PDF
- Abbott’s FreeStyle Libre® 3 receives U.S. FDA clearance - features world’s smallest, thinnest and most accurate 14-day glucose sensor. Press release. Abbott; May 31, 2022. Accessed June 6, 2022. https://abbott.mediaroom.com/2022-05-31-Abbotts-FreeStyle-Libre-R-3-Receives-U-S-FDA-Clearance-Features-Worlds-Smallest,-Thinnest-and-Most-Accurate-14-Day-Glucose-Sensor
- Karinka SA, Brazg RL, Castorino KN, et al. Performance of FreeStyle Libre 3 System. Diabetes. 2022;71(suppl 1):76–LB. https://doi.org/10.2337/db22-76-LB
- Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505-537. doi: 10.1016/j.eprac.2021.04.008
- Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11(9):e5634. doi: 10.7759/cureus.5634
- Draznin B, Aroda VR, Bakris G, et al; American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range targets for. Diabetes Care. 2019;42(8):1593-1603. doi: 10.2337/dci19-0028
- Bergenstal RM. Understanding continuous glucose monitoring data. Role of Continuous Glucose Monitoring in Diabetes Treatment. American Diabetes Association. 2018:20-23.
- Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314-316. doi: 10.1089/dia.2017.0455
- Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37-46. doi: 10.1016/j.diabres.2017.12.015
- Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280. doi: 10.2337/dc18-1581
- Klimontov VV, Saik OV, Korbut AI. Glucose variability: how does it work? Int J Mol Sci. 2021;22(15):7783. doi: 10.3390/ijms22157783
- Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221-230. doi: 10.1016/S2213-8587(18)30136-0
- El Malahi A, Van Elsen M, Charleer S, et al. Chronic complications versus glycaemic variability, time in range and HbA1c in people with type 1 diabetes: sub study of the RESCUE-trial. Endocrine Abstracts. 2020;71(012). doi: 10.1530/endoabs.71.012
- Berganstal RM, Hachman-Nielsen E, Kvist K, Buse JB. Derived time-in-range is associated with MACE in T2D: data from the DEVOTE trial. Diabetes. 2020;69(suppl 1). doi: 10.2337/db20-21-LB
- UK Hypoglycemia Study Group. Risk of hypoglycemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetalogia. 2007;50(6):1140-1147. doi: 10.1007/s00125-007-0599-y
- Choudhary P, Amiel SA. Hypoglycaemia: current management and controversies. Postgrad Med J. 2011;87(1026):298-306. doi: 10.1136/pgmj.2008.06819
- Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. Risk factors for severe hypoglycemia in black and white adults with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes Care. 2017;40(12):1661-1667. doi: 10.2337/dc17-0819
- Punthakee Z, Miller ME, Launer LJ, et al; ACCORD Group of Investigators, ACCORD-MIND Investigators. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787-793. doi: 10.2337/dc11-1855
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410-1418. doi: 10.1056/NEJMoa1003795
- Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. doi: 10.1186/s13098-020-00529-z
- Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol. 2019;13(3):575-583. doi: 10.1177/1932296818812062
- Reiterer F, Polterauer P, Schoemaker M, et al. Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol. 2017;11(1):59-67. doi: 10.1177/1932296816662047
- Ziegler R, von Sengbusch S, Kröger J, et al. Therapy adjustments based on trend arrows using continuous glucose monitoring systems. J Diabetes Sci Technol. 2019;13(4):763-773. doi: 10.1177/1932296818822539
- Beck RW, Riddlesworth T, Ruedy K, et al; DIAMOND Study Group. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. doi: 10.1001/jama.2016.19975
- Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people - a less well recognized risk factor for frailty. Aging Dis. 2015;6(2):156-167. doi: 10.14336/ad.2014.0330
- Aleppo G, Ruedy KJ, Riddlesworth TD, et al; REPLACE-BG Study Group. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40(4):538-545. doi: 10.2337/dc16-2482
- Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a random-ized trial. Ann Intern Med. 2017;167(6):365-374. doi: 10.7326/m16-2855
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011;34(11):2403-2405. doi: 10.2337/dc11-1248
- Choudhary P, Olsen BS, Conget I, Welsh JB, Worrink L, Shin JJ. Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther. 2016;18(5):288-291. doi: 10.1089/dia.2015.0324
- Cohen RM, Holmes YR, Chenier TC Joiner CH. Discordance between HbA1c and fructosamine: evi-dence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care. 2003;26(1):163-167. doi: 10.2337/diacare.26.1.163
- Kuenen JC, Borg R, Kuik DJ, et al; ADAG Study Group. Does glucose variability influence the relation-ship between mean plasma glucose and HbA1c levels in type 1 and type 2 diabetic patients? Diabetes Care. 2011;34(8):1843-1847. doi: 10.2337/dc10-2217
- Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. doi: 10.1001/jama.2016.19976
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised con-trolled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805
- Polonsky WH, Hessler D, Ruedy KJ, Beck RW; DIAMOND Study Group. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40(6):736-741. doi: 10.2337/dc17-0133
- Šoupal J, Petruželková L, Flekač M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study. Diabetes Technol Ther. 2016;18(9):532-538. doi: 10.1089/dia.2016.0171
- van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (in control): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893-902. doi: 10.1016/s2213-8587(16)30193-0
- Munshi M, Slyne C, Davis D, et al. Use of technology in older adults with type 1 diabetes: clinical characteristics and glycemic metrics. Diabetes Technol Ther. 2022;24(1):1-9. doi: 10.1089/dia.2021.0246
- Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing tech-nology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. doi: 10.1016/s0140-6736(16)31535-5
- Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayan G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. doi: 10.1007/s13300-016-0223-6
- Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nation-wide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389-397. doi: 10.2337/dc19-1610
- Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care. 2019;7(1):e000809. doi: 10.1136/bmjdrc-2019-000809
- Gilbert TR, Noar A, Blalock O, Polonsky WH. Change in hemoglobin A1c and quality of life with real-time continuous glucose monitoring use by people with insulin-treated diabetes in the landmark study. Diabetes Technol Ther. 2021;23(S1):S35-S39. doi: 10.1089/dia.2020.0666
- Agarwal S, Mathew J, Davis GM, et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care. 2021;44(3):847-849. doi: 10.2337/dc20-2219
- Bellido V, Perez A. Inpatient hyperglycemia management and COVID-19. Diabetes Ther. 2021;12(1):121-132. doi: 10.1007/s13300-020-00966-z
- Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Updated May 31, 2022. Accessed June 2, 2022. www.covid19treatmentguidelines.nih.gov/
- Horby P, Lim SH, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436
- Shen Y, Fan X, Zhang L, et al. Thresholds of glycemia and the outcomes of COVID-19 complicated with diabetes: a retrospective exploratory study using continuous glucose monitoring. Diabetes Care. 2021;44(4):976-982. doi: 10.2337/dc20-1448
- Carlson AL, Martens TW, Johnson L, Criego AB. Continuous glucose monitoring integration for remote diabetes management: virtual diabetes care with case studies. Diabetes Technol Ther. 2021;23(S3):S56-S65. doi: 10.1089/dia.2021.0241
- Bergenstal RM, Layne JE, Zisser H, et al. Remote application and use of real-time continuous glucose monitoring by adults with type 2 diabetes in a virtual diabetes clinic. Diabetes Technol Ther. 2021;23(2):128-132. doi: 10.1089/dia.2020.0396
- Gal RL, Cohen NJ, Kruger D, et al. Diabetes telehealth solutions: improving self-management through remote initiation of continuous glucose monitoring. J Endocr Soc. 2020;4(9):bvaa076. doi: 10.1210/jendso/bvaa076
- Vigersky RA, Velado K, Zhong A, Agrawal P, Cordero TL. The effectiveness of virtual training on the MiniMed™ 670G system in people with type 1 diabetes during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(2):104-109. doi: 10.1089/dia.2020.0234
- Burckhardt MA, Abraham MB, Mountain J, et al. Improvement in psychosocial outcomes in children with type 1 diabetes and their parents following subsidy for continuous glucose monitoring. Diabetes Technol Ther. 2019;21(10):575-580. doi: 10.1089/dia.2019.0149
- Dixon RF, Zisser H, Layne JE, et al. A virtual type 2 diabetes clinic using continuous glucose monitor-ing and endocrinology visits. J Diabetes Sci Technol. 2020;14(5):908-911. doi: 10.1177/1932296819888662
- Frielitz FS, Müller-Godeffroy E, Hübner J, et al. Monthly video-consultation for children with type 1 diabetes using a continuous glucose monitoring system: design of ViDiKi, a multimethod intervention study to evaluate the benefit of telemedicine. J Diabetes Sci Technol. 2020;14(1):105-111. doi: 10.1177/1932296819861991
- Kamusheva M, Tachkov K, Dimitrova M, et al. A systematic review of collective evidences investi-gating the effect of diabetes monitoring systems and their application in health care. Front Endocrinol (Lausanne). 2021;12:636959. doi: 10.3389/fendo.2021.636959
- Polsky S, Garcetti R, Pyle L, Joshee P, Demmitt JK, Snell-Bergeon JS. Continuous glucose monitor use with and without remote monitoring in pregnant women with type 1 diabetes: a pilot study. PLoS One. 2020;15(4):e0230476. doi: 10.1371/journal.pone.0230476
