- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Comparative Effectiveness of Larotrectinib and Entrectinib for TRK Fusion Cancer
ABSTRACT
Larotrectinib and entrectinib are tumor-agnostic tropomyosin receptor kinase (TRK) inhibitors that are indicated for the treatment of advanced or metastatic solid tumor cancers with neurotrophic tyrosine receptor kinase (NTRK) gene fusions. Regulatory approval of both agents was based on data from single-arm phase 1/2 studies, including tumor-agnostic basket trials. In the absence of randomized controlled trials, there remains a paucity of data to demonstrate the comparative effectiveness of larotrectinib and entrectinib vs established standard-of-care treatments in cancers with NTRK gene fusions. Furthermore, no studies have directly compared the 2 agents. This article reviews what is known about the comparative effectiveness of larotrectinib and entrectinib vs standard therapies in TRK fusion cancer and examines the comparative effectiveness of the 2 TRK inhibitors. Historical and intrapatient comparisons suggest that TRK inhibitors improve disease response compared with preexisting treatments across most tumor histologies; indirect and limited comparisons of phase 1/2 data and preliminary simulation modeling suggest a potential advantage for larotrectinib over entrectinib in terms of clinical response and survival. Although limited, these data provide some insight into the position of these treatments in established treatment paradigms for TRK fusion cancer, a setting where real-world evidence will be slow to accrue due to the rare nature of these tumors but may be the only way in the future to answer the outstanding questions regarding these 2 agents. Meanwhile, we need to try to obtain the maximum benefit that can be achieved for our patients using the currently available knowledge.
Am J Manag Care. 2022;28(suppl 2):S26-S32. https://doi.org/10.37765/ajmc.2022.88845
For author information and disclosures, see end of text.
Introduction
Larotrectinib and entrectinib are tumor-agnostic treatments for advanced or metastatic solid tumors harboring neurotrophic tyrosine receptor kinase (NTRK) gene fusions. NTRK gene fusions are oncogenic drivers in a diverse range of tumor types, including lung cancer, colorectal cancer, thyroid cancer, salivary gland cancer, and sarcomas. These gene alterations are present in tumors of both adults and children.1 Although NTRK gene fusions are generally rare, occurring in less than 1% of all cancers overall,1,2 they are highly prevalent in certain rare tumors (eg, secretory breast carcinoma, secretory carcinoma of the salivary gland, infantile fibrosarcoma, and congenital mesoblastic nephroma).3 The rarity of NTRK gene fusions, combined with the diverse range of tumor types in which they are found and the heterogeneity of tumor-specific standard-of-care treatments, makes the clinical evaluation of tropomyosin receptor kinase (TRK) inhibitors challenging and the use of gold-standard randomized controlled trials unfeasible.4-6
Drug development programs have adapted to integrate biomarker-driven tumor-agnostic approaches into trial design. Indeed, the TRK inhibitors larotrectinib and entrectinib were among the earliest cancer drugs to receive tumor-agnostic approvals from the US FDA and European Medicines Agency (EMA). Both are approved for use in locally advanced or metastatic TRK fusion cancer that has progressed with standard-of-care therapies and in which no alternative satisfactory treatment options exist or where surgical resection is likely to result in severe morbidity. Larotrectinib is approved for adults and pediatric patients 1 month or older. Entrectinib is approved for adults and pediatric patients 12 years or older.7-10
Regulatory approvals for both larotrectinib and entrectinib were based on findings from single-arm phase 1/2 studies, including basket trials that enrolled patients based on the presence of NTRK gene fusions, regardless of age or tumor type.11-13 Despite regulatory acceptance of this adaptive trial approach, there remains a paucity of comparative effectiveness data for TRK inhibitors vs established standard-of-care treatments, which presents a challenge to their integration into routine clinical practice. In addition, no studies have directly compared the effects of larotrectinib and entrectinib in TRK fusion cancer.
The American Society of Clinical Oncology and European Society for Medical Oncology clinical practice guidelines recommend either larotrectinib or entrectinib as treatment options for solid tumors with NTRK gene fusions, including breast, non–small cell lung cancer (NSCLC), soft tissue sarcoma, salivary, and thyroid14-19; however, these guidelines provide no indication of the relative effectiveness of the 2 agents. Furthermore, switching from one TRK inhibitor to another has not been investigated and is not recommended due to the likely development of mechanisms of cross-resistance, which can include the appearance of resistance mutations in these genes.12,20,21 Thus, the initial decision between the 2 drugs represents the only opportunity to choose the most appropriate first-generation TRK inhibitor for each individual patient.
In this article, we review the comparative effectiveness of larotrectinib, entrectinib, and non–TRK-targeted standard-of-care therapies for TRK fusion cancer, utilizing indirect comparisons that aimed to overcome the challenge of translating tumor-agnostic basket trial data into clinical practice.
Efficacy of TRK Inhibitors vs Standard-of-Care Therapies
The initial FDA approval of larotrectinib for TRK fusion cancer was based on a pooled analysis of patients from 3 phase 1/2 trials (including a basket trial) in adult, adolescent, and pediatric patients: an adult phase 1 study (NCT02122913); the phase 1/2 SCOUT study (NCT02637687); and the phase 2 NAVIGATE basket study (NCT02576431). In the first publication of data from these trials (in 55 patients with 17 different tumors), the investigator-assessed objective response rate (ORR) was 80%.12 In a more recent analysis carried out in an expanded population of 218 patients, the ORR was 75%, with a median duration of response (DOR) of 49.3 months and a median progression-free survival (PFS) of 35.4 months. Responses were seen regardless of tumor type. Median overall survival (OS) had not been reached after a median follow-up of 22.3 months. The 36-month OS rate was 77%.22
The regulatory data set for the US approval of entrectinib was based on a pooled analysis of 54 adult patients with solid tumors and NTRK gene fusions from 3 single-arm phase 1/2 trials: ALKA-372-001 (EudraCT 2012–000148–88), STARTRK-1 (NCT02097810), and STARTRK-2 (NCT02568267).11 The European approval was based on an expanded data set of 74 patients.10 Entrectinib was associated with these responses: ORR, 57%; median DOR, 10.4 months; median PFS, 11.2 months; and median OS, 21 months.11 The STARTRK-NG trial subsequently provided evidence of tumor response and durable disease control with entrectinib in adolescent and pediatric patients.13,23 The clinical data for larotrectinib and entrectinib are reviewed in more detail by Kummar et al in this supplement.
Although basket trials offer an adaptive tumor-agnostic solution and were requested for regulatory purposes to provide evidence of efficacy and safety of both larotrectinib and entrectinib, some questions remain regarding the translation of these findings to clinical practice. The very nature of the basket trial adds patient-level histological heterogeneity, with many individual and rare tumor types grouped together due to their common oncogenic driver. Widely different prognoses across cancers of different origins, along with the absence of a single standard-of-care treatment across distinct tumor histologies, confound interpretation and extrapolation of time-to-event outcomes (eg, survival) from these studies to the clinic.6 Nevertheless, it is crucial that clinicians and decision makers understand the place of tumor-agnostic therapies in the established treatment paradigm for specific cancers. Historical data comparisons for specific tumor histologies and intrapatient comparisons are 2 methods that have been used to indirectly compare the clinical efficacy of larotrectinib, entrectinib, and standard-of-care treatment.
Comparative Effectiveness vs Historical Standard Therapies
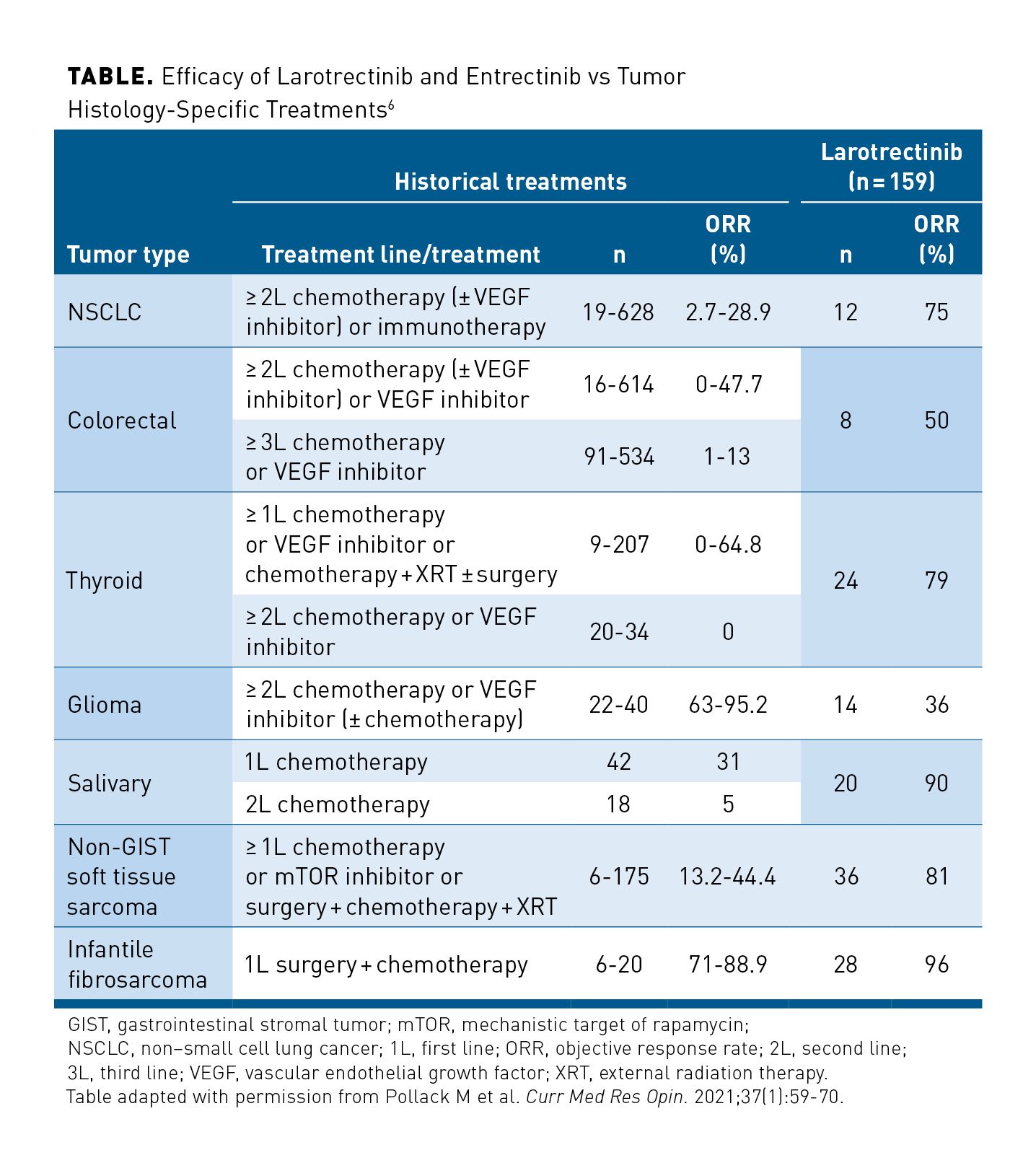
Pollack and colleagues indirectly compared historical treatment data for advanced-stage/metastatic solid tumor histologies known to harbor NTRK gene fusions (NSCLC, colorectal cancer, thyroid cancer, gliomas, soft tissue sarcoma, salivary gland cancer, and infantile fibrosarcoma) with pooled phase 1/2 data from the 3 larotrectinib trials to provide a side-by-side comparison of efficacy.6 Despite small patient numbers, the ORR for larotrectinib was higher than that reported previously for historical standard treatments across most specific tumor histologies, especially in later lines of therapy where larotrectinib is most likely to be used (Table).6 This is not surprising in that the efficacy of chemotherapy and other systemic treatments decreases with subsequent lines of therapy, as the disease becomes more resistant.24,25 In NSCLC, for instance, the observed ORR for larotrectinib was 75% compared with up to 29% for second-line or subsequent historical treatment. Similar trends were observed for colorectal cancer, soft tissue sarcomas, and salivary gland cancers. Although the findings of these comparisons are of interest, it must be noted that these were general comparisons of outcomes observed in a small number of patients from the larotrectinib trials. No matching for demographic or clinical variables was performed, and outcomes were compared with an unselected historical tumor histology–based population receiving standard therapies in which NTRK gene fusion status was unknown. However, retrospective real-world database analyses have demonstrated that NTRK gene fusion status has no prognostic impact across various tumor types in the absence of TRK-targeted therapies.26,27
Intrapatient Comparison
Growth modulation index (GMI) analyses may provide a more informative comparison of TRK inhibitor effectiveness vs standard therapy in the setting of rare TRK fusion cancer, overcoming some of the challenges associated with single-arm trials, which do not provide comparative data against a control. Using the GMI provides intrapatient comparative data using individual patients as their own control, thus generating comparative efficacy data for drugs developed in single-arm trials. The end point has been endorsed by the EMA for truly rare tumors or very narrow indications.28
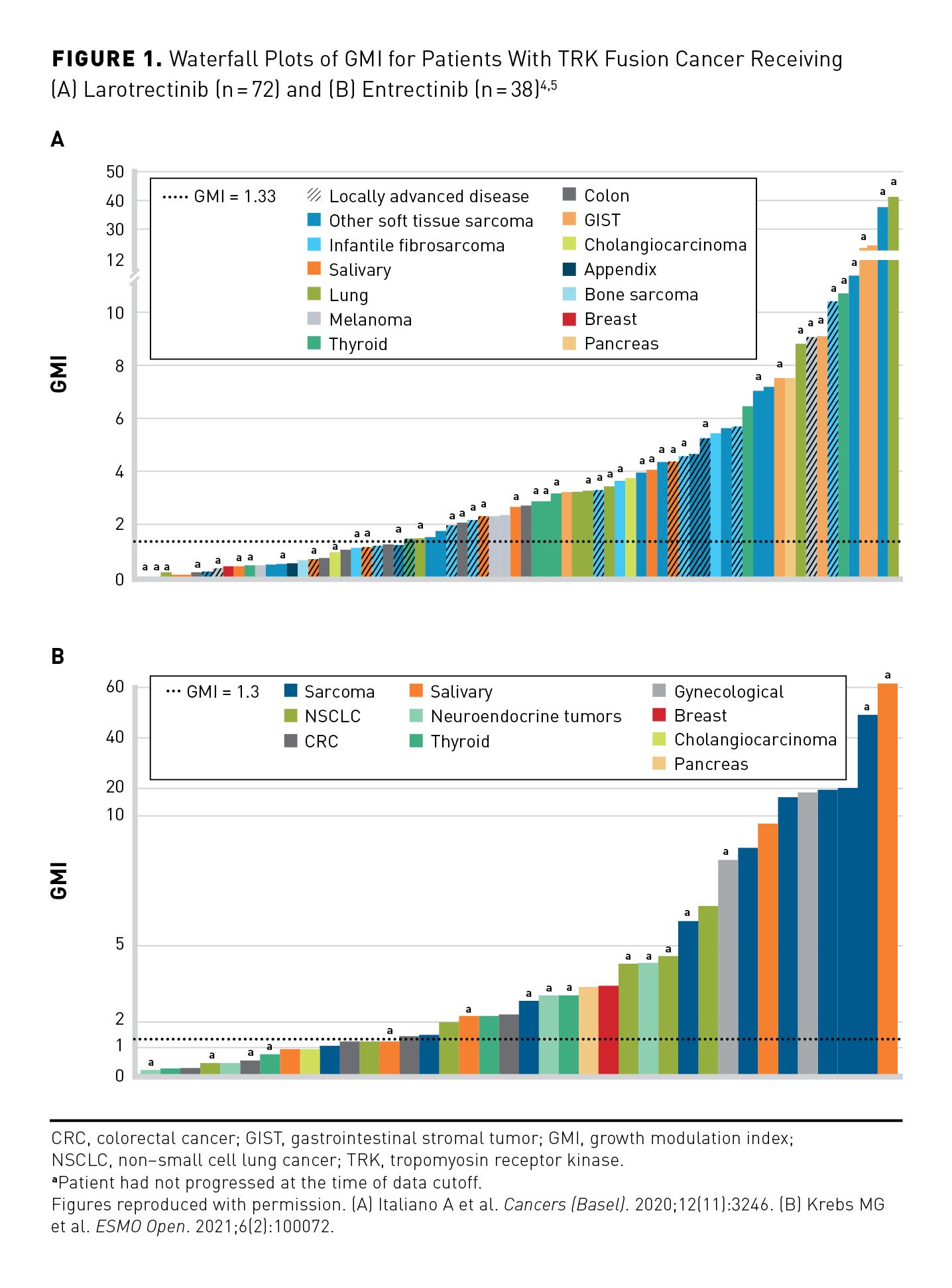
GMI is the ratio of PFS on current therapy to time to progression (TTP) on the most recent prior line of therapy within the same patient. Since TTP typically decreases with each subsequent line of anticancer therapy, a GMI of greater than 1 would suggest that an investigational therapy is having a positive impact on a tumor’s natural history. A GMI of 1.33 or greater indicates an improvement in PFS of at least 33% over the previous line of therapy and has been proposed as a threshold of meaningful clinical activity.29 Separate retrospective exploratory GMI analyses have been performed to determine whether larotrectinib, entrectinib, or both provide clinical benefit vs prior standard-of-care treatment in patients with TRK fusion cancer. Findings from the 2 analyses suggest that both larotrectinib and entrectinib provide improved clinical outcomes in patients with TRK fusion cancer compared with prior therapies.4,5,22
In an initial analysis of 72 patients treated with larotrectinib who had received at least 1 prior line of systemic therapy, median GMI was 2.68—more than double the 1.33 threshold for meaningful clinical activity suggested in the literature—with 65% of patients having a GMI of 1.3 or greater and 26% having a GMI of 5 or greater (Figure 1A).4 Nonparametric analysis adjusting for censored PFS durations indicated a 0.75 probability of GMI of at least 1.33, with Kaplan-Meier estimates demonstrating a median GMI of 6.46. Median PFS on larotrectinib was longer than TTP on the prior therapy (not estimable [NE] vs 3.0 months; HR, 0.220; 95% CI, 0.146-0.332); this supported the GMI results.4 In a more recent expanded data set (n= 140) with additional follow-up, the Kaplan-Meier–estimated median GMI was 8.9. The majority (74%) of patients met the GMI threshold of 1.33 or greater, regardless of age, tumor type, or prior number of therapies. Median PFS on larotrectinib was 33.0 months and median TTP on the prior therapy was 3.0 months (HR, 0.22; 95% CI, 0.16-0.30).22
For entrectinib, GMI analyses were based on data from 71 adults with TRK fusion cancer treated during the single-arm phase 2 STARTRK-2 trial. Among patients who had progressed on prior therapy (n=38), median GMI was 2.53, with 65.8% of patients having a GMI ratio at least 1.3 (Figure 1B).5 The ORR was 60.5% for entrectinib vs 15.8% for prior therapy in this setting, and median PFS for entrectinib exceeded median time to discontinuation for prior therapy (11.2 vs 2.9 months).5
Parametric Extrapolation-Based Comparisons
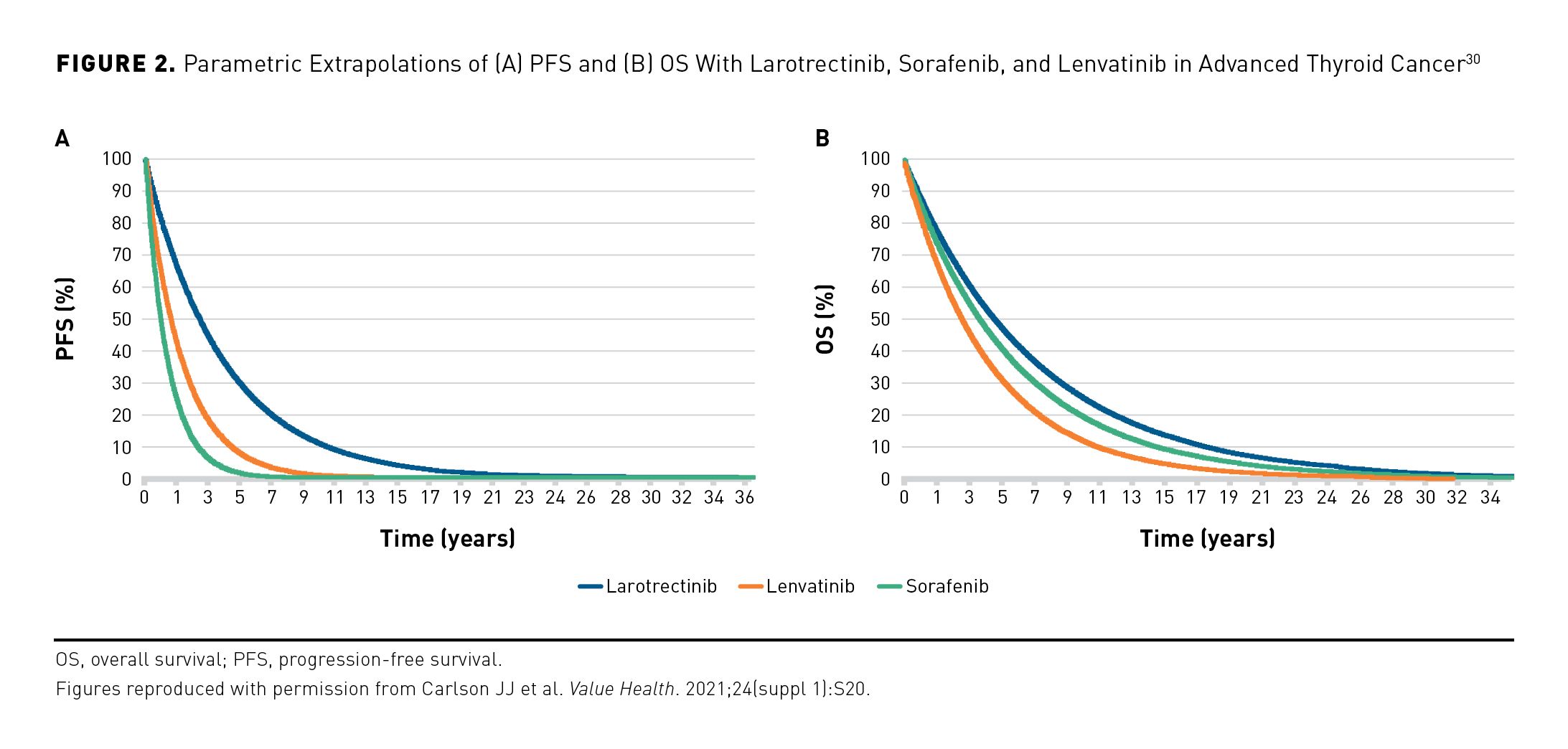
For patients with locally advanced or metastatic radioactive iodine–refractory differentiated thyroid cancer, standard-of-care systemic therapies are the tyrosine kinase inhibitors sorafenib and lenvatinib. The long-term comparative effectiveness of larotrectinib vs these agents for radioactive iodine–refractory advanced thyroid cancer has been evaluated using partitioned survival models that estimate the proportion of patients in the preprogression and progression health states. In this analysis, larotrectinib was estimated to provide improved life-year outcomes compared with sorafenib and lenvatinib (Figure 2).30 In the base case, model-estimated mean preprogression life-years were 4.24 for larotrectinib, 1.35 for sorafenib, and 2.14 for lenvatinib; estimated mean total life-years were 6.32, 5.47, and 4.36, respectively. Larotrectinib was also estimated to provide improved quality-adjusted life-year (QALY) outcomes compared with sorafenib and lenvatinib. Estimated mean preprogression QALYs were 3.57, 1.09, and 1.80, respectively; estimated mean total QALYs were 4.61, 3.15, and 2.91, respectively.30 In this analysis, NTRK gene fusion status in patients receiving lenvatinib and sorafenib was unknown.
Modeling the Comparative Effectiveness of Larotrectinib and Entrectinib
To date, no study has compared larotrectinib with entrectinib for the treatment of solid tumors with NTRK gene fusions. Although these agents were both studied in similar basket trials, there are important differences between the larotrectinib and entrectinib clinical trial patient populations that make direct comparison of the 2 data sets challenging.11,31
Matching-Adjusted Indirect Comparison
The relative effectiveness of larotrectinib and entrectinib for the treatment of patients with TRK fusion cancer has been evaluated using a matching-adjusted indirect comparison. In this analysis, adult patients from larotrectinib clinical trials and from published aggregate entrectinib trial data were identified and matched based on available common baseline demographic and clinical characteristics that may be predictive of outcome (eg, gender, age, race, ECOG performance status, select tumor types, metastatic disease, NTRK gene, central nervous system metastases, number of prior lines of therapy). After matching, larotrectinib was associated with significantly longer OS compared with entrectinib: median not reached (95% CI, 38.7 months to NE) vs 23.9 months (95% CI, 16.0 months to NE; P<.05).32
Larotrectinib and entrectinib have both been shown to be generally well tolerated in clinical trials. Dose reductions and treatment discontinuations due to treatment-related adverse events (TRAEs) occurred in 8% and 2% of patients receiving larotrectinib respectively,31 and in 27% and 4% of patients receiving entrectinib respectively.11 In a matching-adjusted indirect comparison, serious TRAEs occurred in 6.2% and 10.0% of patients receiving larotrectinib and entrectinib, respectively, and TRAEs leading to discontinuation occurred in 0.5% and 4.0% of patients.32
Parametric Extrapolation-Based Comparisons
In the absence of direct comparative studies or meta-analyses, simulation-based modeling, which extrapolates data from small clinical samples, may offer some insight into how larotrectinib and entrectinib compare and may help inform clinical and health care system reimbursement decision-making.
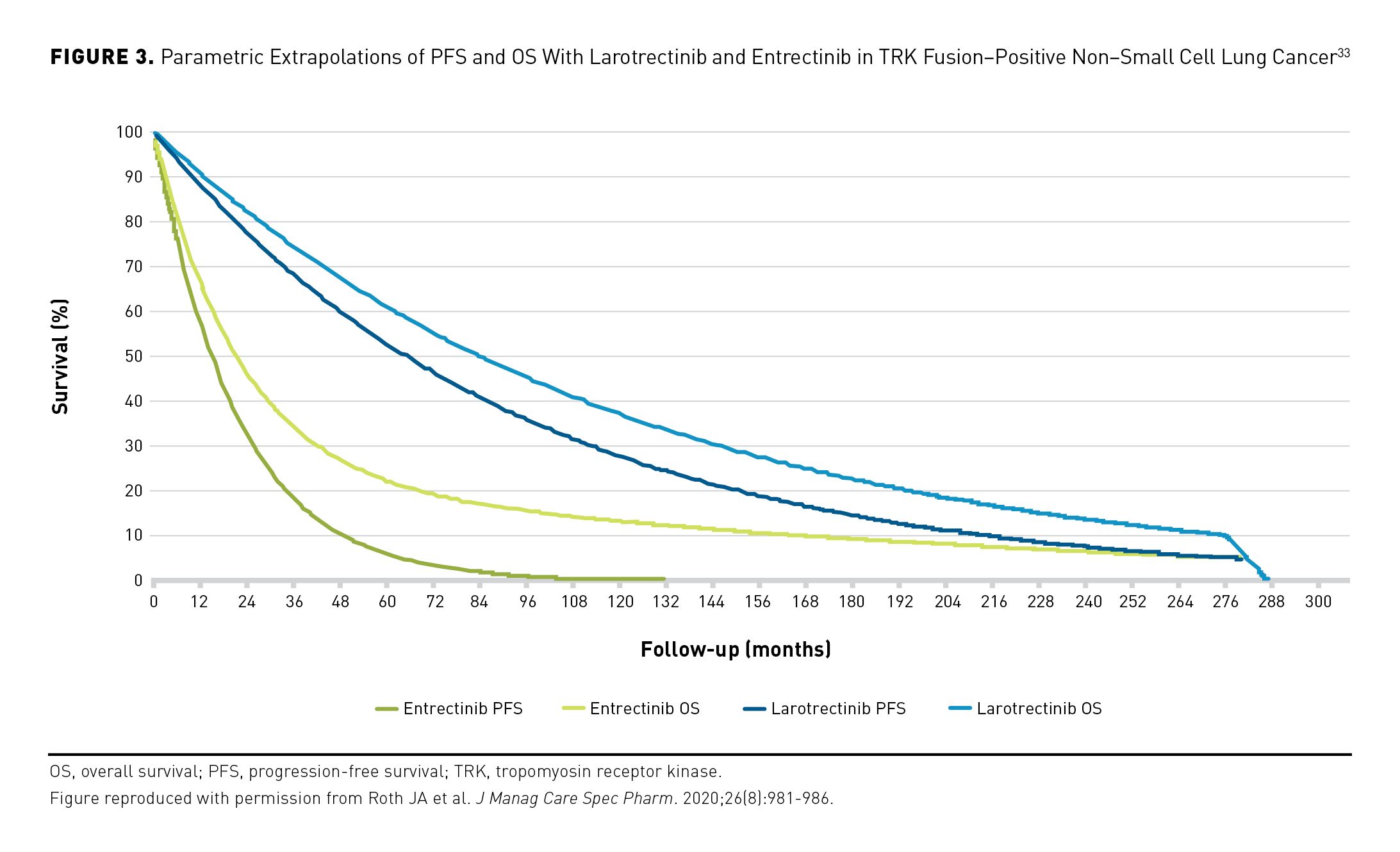
Roth and colleagues developed a partitioned survival model to project the long-term comparative effectiveness of larotrectinib vs entrectinib in second-line treatment of metastatic NSCLC. The authors performed parametric extrapolations of in-trial PFS and OS data (median follow-up, 13 months) from patients with TRK fusion–positive NSCLC treated with larotrectinib (n=12) and entrectinib (n=10) during single-arm phase 1/2 studies. Larotrectinib was estimated to provide improved life-year outcomes compared with entrectinib (Figure 3).33 In the base case, model-estimated mean preprogression life-years were 7.5 for larotrectinib and 1.9 for entrectinib; estimated mean total life-years were 9.2 and 4.4, respectively. Larotrectinib was also estimated to provide improved QALY outcomes compared with entrectinib. Estimated mean preprogression QALYs were 5.0 and 1.2, respectively, with respective estimated mean total QALYs of 5.8 and 2.4. Sensitivity analyses indicated persistent gains for larotrectinib under a range of clinically plausible survival effects.33
This approach has also been used to model the comparative effectiveness of larotrectinib vs entrectinib in patients with TRK fusion–positive solid tumors with brain metastases. This analysis included 14 larotrectinib-treated patients (7 NSCLC, 4 thyroid cancer, 2 melanoma, 1 breast cancer) and 16 entrectinib-treated patients (8 NSCLC, 4 thyroid cancer, 2 sarcoma, 1 breast cancer, 1 salivary gland cancer). Larotrectinib was estimated to provide improved life-year outcomes compared with entrectinib in these patients. Estimated mean preprogression life-years were 1.91 for larotrectinib and 0.80 for entrectinib; mean total life-years were 2.76 and 1.66, respectively.34
Conclusions
Regulatory acceptance of single-arm trial data led to the tumor-agnostic approvals of the TRK inhibitors larotrectinib and entrectinib for treatment of solid tumors harboring NTRK gene fusions. Historical and intrapatient comparisons, in addition to parametric extrapolation-based modeling, suggest that TRK inhibitors improve disease response and survival compared with non–TRK-targeted standard therapies across most tumor histologies. Matching-adjusted indirect comparison and simulation studies suggest greater benefit to survival outcomes with larotrectinib compared with entrectinib. Although limited due to the number of patients and lack of direct comparisons, these analyses provide some insight into the position of TRK inhibitors in established treatment paradigms in a setting where real-world evidence will be slow to gather due to the rare nature of these cancers. This carries importance because it is likely that patients will have only 1 opportunity to receive a first-generation TRK inhibitor before the development of resistance mutations, or other mechanisms of resistance, that a second first-generation TRK inhibitor would then be ineffective against.
Acknowledgments
Medical writing support was provided by Michael Sheldon, PhD, and editorial support was provided by George Chappell, MSc, both of Scion (London, UK), supported by Bayer according to Good Publication Practice guidelines. Bayer was involved in fact-checking information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Author affiliations: Thomas Jefferson University Hospitals (MSB); University of Washington (JJC, SDS); University of California, Los Angeles (NF); Institut Bergonié (AI); Oregon Health and Science University (SK); Rigshospitalet (UL); Fred Hutchinson Cancer Research Center (SDS).
Funding source: This article was published as part of a supplement that was financially supported by Bayer HealthCare Pharmaceuticals, Inc. Noah Federman’s research is supported by NIH National Center for Advancing Translational Science (NCATS) UCLA Clinical & Translational Science Institute (CTSI) Grant Number UL1TR001881.
Author disclosures: Dr Brose, Dr Carlson, Dr Federman, Dr Kummar, Dr Lassen, and Dr Sullivan have been part of consultancies or paid advisory boards with Bayer. Dr Brose and Dr Federman have been part of consultancies or paid advisory boards with Loxo Oncology. Dr Brose has also been part of consultancies or paid advisory boards with Genentech, AstraZeneca, and Eli Lilly and Company. Dr Brose has received honoraria from Clinical Care Options, Medscape, Onclive®, and PeerView. Dr Brose has also disclosed that research support to the University of Pennsylvania School of Pharmacy has been provided by Bayer, Loxo Oncology, Genentech, Eisai, Blueprint Medicines, Eli Lilly and Company, and Novartis. Dr Carlson has received honoraria from Adaptive Biotechnologies. Dr Federman has received lecture fees for speaking at the invitation of Bayer and received patent royalties from NanoValent Pharmaceuticals, Inc. Dr Federman has also disclosed that he holds stock in the for-profit health care companies Genmab, Reata Pharmaceuticals, and bluebird bio, Inc. Dr Italiano and Dr Lassen have both received grants from Roche. Dr Italiano has also received grants from AstraZeneca, Bayer, Merck, Merck Sharp & Dohme, and PharmaMar. Dr Italiano has disclosed that he has received honoraria from Bayer, Daiichi Sankyo, Epizyme, Ipsen, Roche, and SpringWorks Therapeutics. Dr Kummar has been part of consultancies or paid advisory boards with Boehringer Ingelheim, SpringWorks Therapeutics, Gilead, EcoR1 Capital, Seagen, Mundipharma, Mirati Therapeutics, Genome & Company, and Harbour Biomed. Dr Kummar has also disclosed that she is the cofounder and is an equity holder for PathomIQ. Dr Kummar has also disclosed that she is the editor-in-chief of Current Problems in Cancer (Elsevier) and that her spouse is a scientific adviser for Cadila Pharmaceuticals Ltd and is the founder of Arxeon Inc. Dr Lassen has also reported that he has been part of consultancies or paid advisory boards with Pfizer and Novartis. Dr Lassen has also received grants from Bristol Myers Squibb, GlaxoSmithKline, and Pfizer.
Authorship information: Analysis and interpretation of data (JCC, AI, MSB, NF, UL, SK, SDS); drafting of the manuscript (JCC, AI, MSB, NF, UL, SK, SDS); critical revision of the manuscript for important intellectual content (JCC, AI, MSB, NF, UL, SK, SDS).
Address correspondence to: Josh J. Carlson, PhD, MPH; carlsojj@gmail.com
REFERENCES
1. Cocco E, Scaltriti M, Drilon A. NTRK fusion–positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731-747. doi:10.1038/s41571-018-0113-0
2. Amatu A, Sartore-Bianchi A, Bencardino K, Pizzutilo EG, Tosi F, Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol. 2019;30(Suppl 8):viii5-viii15. doi:10.1093/annonc/mdz383
3. Federman N, McDermott R. Larotrectinib, a highly selective tropomyosin receptor kinase (TRK) inhibitor for the treatment of TRK fusion cancer. Expert Rev Clin Pharmacol. 2019;12(10):931-939. doi:10.1080/ 17512433.2019.1661775
4. Italiano A, Nanda S, Briggs A, et al. Larotrectinib versus prior therapies in tropomyosin receptor kinase fusion cancer: an intra-patient comparative analysis. Cancers (Basel). 2020;12(11):3246. doi:10.3390/ cancers12113246
5. Krebs MG, Blay J-Y, Le Tourneau C, et al. Intrapatient comparisons of efficacy in a single-arm trial of entrectinib in tumour-agnostic indications. ESMO Open. 2021;6(2):100072. doi:10.1016/j.esmoop.2021.100072
6. Pollack M, Keating K, Wissinger E, Jackson L, Sarnes E, Cuffel B. Transforming approaches to treating TRK fusion cancer: historical comparison of larotrectinib and histology-specific therapies. Curr Med Res Opin. 2021;37(1):59-70. doi:10.1080/03007995.2020.1847057
7. Bayer AG. Vitrakvi: annex I – summary of product characteristics. European Medicines Agency; updated May 2021. Accessed October 1, 2021. https://www.ema.europa.eu/en/documents/product-information/ vitrakvi-epar-product-information_en.pdf
8. Vitrakvi. Prescribing information. Bayer HealthCare Pharmaceuticals; updated March 2021. Accessed November 15, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210861s006lbl.pdf
9. Rozlytrek. Prescribing information. Genentech; updated November 2021. Accessed November 15, 2021. https://www.gene.com/download/pdf/rozlytrek_prescribing.pdf
10. Roche Pharma AG. Rozlytrek: annex I – summary of product characteristics. European Medicines Agency; updated September 2021. Accessed October 1, 2021. https://www.ema.europa.eu/en/documents/ product-information/rozlytrek-epar-product-information_en.pdf
11. Doebele RC, Drilon A, Paz-Ares L, et al; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion–positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
12. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
13. Robinson GW, Gajjar AJ, Gauvain KM, et al. Phase 1/1B trial to assess the activity of entrectinib in children and adolescents with recurrent or refractory solid tumors including central nervous system (CNS) tumors. J Clin Oncol. 2019;37(suppl 15):abstr 10009. doi:10.1200/JCO.2019.37.15_suppl.10009
14. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623-1649. doi:10.1016/j.annonc.2020.09.010
15. Planchard D, Popat S, Kerr K, et al; ESMO Guidelines Committee. Metastatic non–small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192-iv237. doi:10.1093/annonc/mdy275
16. Filetti S, Durante C, Hartl D, et al; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856-1883. doi:10.1093/annonc/mdz400
17. Geiger JL, Ismaila N, Beadle B, et al. Management of salivary gland malignancy: ASCO Guideline. J Clin Oncol. 2021:39(17):1909-1941. doi:10.1200/JCO.21.00449
18. Gronchi A, Miah AB, Dei Tos AP, et al. ESMO Guidelines Committee, EURACAN and GENTURIS. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(11):1348-1365. doi:10.1016/j.annonc.2021.07.006
19. Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO and OH (CCO) Joint Guideline update. J Clin Oncol. 2021;39(9):1040-1091. doi:10.1200/JCO.20.03570
20. Cocco E, Schram AM, Kulick A, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med. 2019;25(9):1422-1427. doi:10.1038/s41591-019-0542-z
21. Drilon A, Ou S-HI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 2018;8(10):1227-1236. doi:10.1158/2159-8290.CD-18-0484
22. Hong DS, Italiano A, Briggs A, et al. Intra-patient comparison from larotrectinib clinical trials in TRK fusion cancer: an expanded dataset. J Clin Oncol. 2021;39(suppl 15):abstr 3114. doi:10.1200/ JCO.2021.39.15_suppl.3114
23. Desai AV, Robinson GW, Basu EM, et al. Updated entrectinib data in children and adolescents with recurrent or refractory solid tumors, including primary CNS tumors. J Clin Oncol. 2020;38(suppl 15):abstr 107. doi:10.1200/JCO.2020.38.15_suppl.107
24. Park IH, Lee KS, Ro J. Effects of second and subsequent lines of chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2015;15(1):e55-62. doi:10.1016/j.clbc.2014.09.001
25. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229-237. doi:10.1200/JCO.2004.05.113
26. Bazhenova L, Lokker A, Snider J, et al. TRK fusion cancer: patient characteristics and survival analysis in the real-world setting. Target Oncol. 2021;16(3):389-399. doi:10.1007/s11523-021-00815-4
27. Bridgewater J, Jiao X, Parimi M, et al. Abstract 394: prognosis and molecular characteristics of patients with TRK fusion cancer in the 100,000 Genomes Project. Cancer Res. 2021;81(13 suppl). doi:10.1158/1538-7445.AM2021-394
28. Guideline on the evaluation of anticancer medicinal products in man. European Medicines Agency. September 22, 2017. Accessed September 9, 2019. https://www.ema.europa.eu/en/documents/scientificguideline/guideline-evaluation-anticancer-medicinal-products-man-revision-5_en.pdf
29. Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs—twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res. 1998;4(5):1079-1086.
30. Carlson JJ, Suh K, Xia F, Williamson T, Sullivan SD. PCN11 The potential long-term comparative effectiveness of larotrectinib vs. lenvatinib or sorafenib for treatment of NTRK fusion–positive metastatic thyroid cancer. Value Health. 2021;24(suppl 1):S20. doi:10.1016/j.jval.2021.04.103
31. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion–positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531-540. doi:10.1016/S1470-2045(19)30856-3
32. Garcia-Foncillas J, Bokemeyer C, Italiano A, et al. 104P Matching-adjusted indirect comparison for treatment of NTRK fusion cancer with larotrectinib versus entrectinib. Ann Oncol. 2021;32(suppl 5):S401. doi:10.1016/j.annonc.2021.08.384
33. Roth JA, Carlson JJ, Xia F, Williamson T, Sullivan SD. The potential long-term comparative effectiveness of larotrectinib and entrectinib for second-line treatment of TRK fusion–positive metastatic lung cancer. J Manag Care Spec Pharm. 2020;26(8):981-986. doi:10.18553/jmcp.2020.20045
34. Suh K, Carlson JJ, Xia F, Williamson T, Sullivan SD. C35 A comparison of extrapolated survival estimates for larotrectinib vs entrectinib in NTRK fusion–positive patients with brain metastasis. J Manag Care Spec Pharm. 2021;27(suppl 10a):S31. doi:10.18553/jmcp.2021.27.issue-10-a

