- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Addressing the Unmet Needs of Patients With Chronic Cough
Overview of Cough
Cough is one of the most common reasons for visits to a medical provider in the United States.1 In 2012, chronic cough accounted for approximately 10% to 38% of visits to US respiratory outpatient practices.2 Cough may be a symptom of a treatable underlying condition; however, in 5% to 10% of patients with chronic cough, there is no identifiable cause.3,4 Patients with chronic cough have considerable health care resource utilization and poor quality of life.3,5,6 As of October 2020, no agents have been approved by the FDA for the treatment of chronic cough. However, several agents are in various stages of development, and preliminary phase 3 results for 1 of these agents are available.7
Cough is a defense reflex to clear the upper airways.2 Cough can be triggered by mechanical or inflammatory changes and by inhalation of mechanical and chemical irritants.2 Cough receptors located at the airway bifurcations, larynx, and distal esophagus provide input through cough afferent pathways, including the superior laryngeal and vagus nerves, to the cerebral cortex and cough center. Efferent pathways then coordinate the muscle response to produce a cough
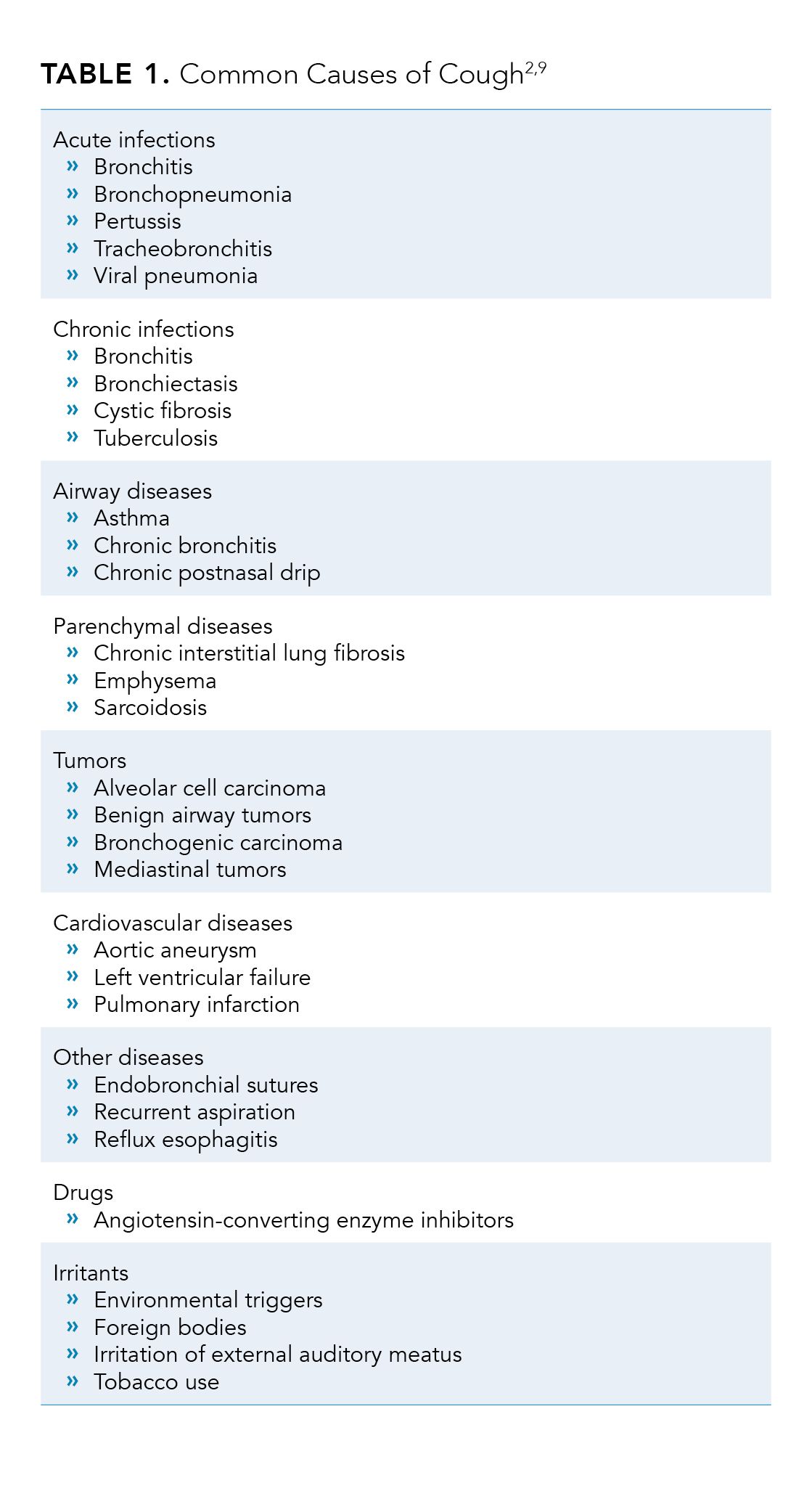
(inhalation, contraction of the abdominal muscles, diaphragm, and chest wall).2,8 Common causes of cough are summarized in Table 1.2,9
Chronic Cough
In adults, chronic cough is generally defined as a cough lasting more than 8 weeks.10,11 In contrast, acute cough is defined as a cough lasting less than 3 weeks.10 Although acute cough is usually self-limiting, chronic cough can be difficult to control despite a clear diagnosis and can impair patients’ quality of life.12
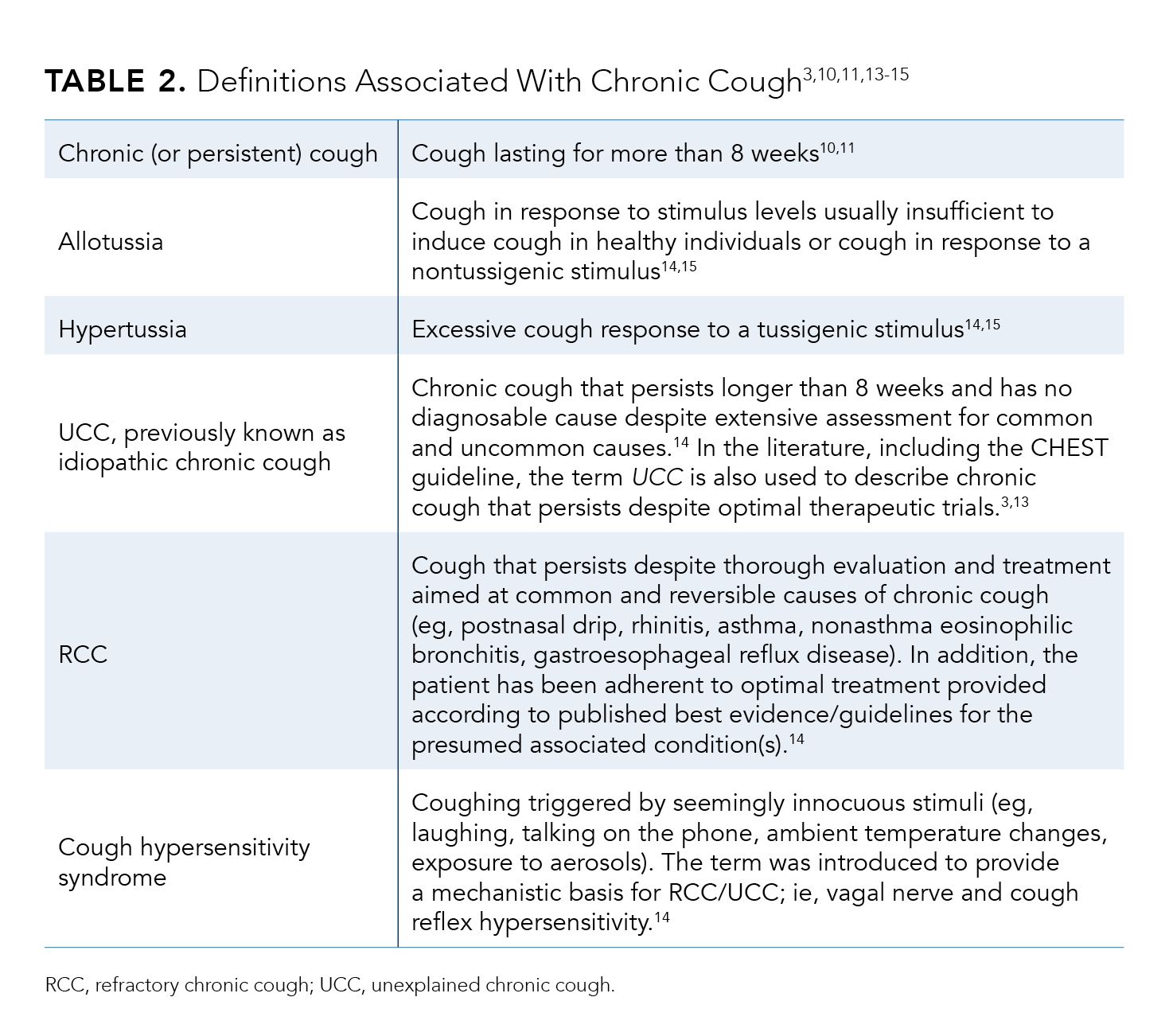
Table 23,10,11,13-15 summarizes various terminologies for chronic cough. The American College of Chest Physicians (ACCP) defines unexplained chronic cough (UCC) as a cough lasting longer than 8 weeks that cannot be explained after thorough investigation including guideline-based therapeutic trials.3 The terms refractory chronic cough (RCC) and unexplained chronic cough (UCC) are often used interchangeably in the literature.3,13 However, some experts advocate for separate definitions for refractory versus unexplained chronic cough.14 “Difficult-to-treat” cough has been used to describe both UCC and RCC, because a chronic cough that is unexplained or does not respond to appropriate therapy is often difficult to treat.3
Clinical Features of Refractory or Unexplained Chronic Cough
Clinical features of RCC/UCC include intermittent bouts of dry cough throughout the day.15 Patients may have allotussia, and coughs may be triggered by nontussive stimuli, including talking or exposure to air conditioning. Hypertussia may also occur, in which patients cough in response to very low levels of tussive stimuli. The cough often arises from the laryngeal area, and laryngeal paresthesia and discomfort can also be triggers. RCC/UCC can persist for months or even years.15
Patient and Health Care System Burden
From 5% to 10% of patients seeking medical attention for a chronic cough have RCC/UCC.3 In the United States and Europe, the prevalence of cough ranges from 9% to 33%.2 Among patients in specialist respiratory clinics, up to 46% of idiopathic coughs are RCC/UCC.2
RCC/UCC is associated with considerable health care resource utilization, including medication use. Patients with chronic cough account for approximately 10% to 38% of visits to respiratory outpatient practice in the United States.2 Within the first year of diagnosis, about 29% of patients with chronic cough will have at least 1 emergency department visit and approximately 10% will have at least 1 hospitalization.5 Additionally, approximately 40% of these patients will have visited at least 2 different specialists. Although only data during the first year after diagnosis are available, increased health care utilization is expected to continue, as 40% of patients, even after various pharmacologic and nonpharmacologic treatment, continue to experience chronic cough.5
Patients with RCC/UCC also have significant impairments in quality of life.3,6 Health-related dysfunction, which was assessed by the Sickness Impact Profile self-administered questionnaire, which included both physical and psychosocial dimensions,6 was significantly correlated with the duration of cough (r = –0.51; P = .001).6 Chronic cough also negatively affects psychosocial dimensions, which are significantly correlated with the duration of cough (r = –0.45; P = .004) and number of cough symptoms (r = 0.44; P < .02).6 In a survey study using the cough-specific quality-of-life questionnaire (CQLQ), mean ± SE CQLQ scores in women (67.1 ± 1.32) and men (59.8 ± 1.86) were higher than historical control without cough (35.06 ± 8.40).16 The physical complications of chronic cough include tissue trauma leading to symptoms including hoarseness; chest pains; and, more rarely, hernias and rib fractures.17 Retching and vomiting, stress incontinence, headaches, and light-headedness are also common. In addition, patients endure sleep deprivation if they have nocturnal coughing.17
Besides adverse impacts on physical health, RCC/UCC also can have marked emotional impacts. Patients feel embarrassment when coughing in the work environment and public places, and friends and colleagues may assume the patient is contagious or is a heavy smoker.17 In a qualitative study, some patients with RCC/UCC perceived that their lives were controlled by their coughing and felt “at the end of the line” with no chance of getting better.18 Chronic cough affects patients’ family members and interpersonal relationships as well. Partners may be forced to sleep in a separate room due to the patient’s nocturnal coughing.17,19 Patients have also detailed frustrating interactions with family members who do not understand the nature of RCC/UCC.20 It is of no surprise that in one survey study, 53% of patients with chronic cough met the diagnostic criteria for depression as measured by the Center for Epidemiologic Studies Depression Scale.21
These physical and emotional tolls can be unceasing, as patients with RCC/UCC experience a chronic cough that persists for many months or years, despite adequate investigation and treatment of identifiable causes.3
Diagnosis of Refractory or Unexplained Cough
It is critical to differentiate RCC/UCC from chronic cough that can be effectively treated because incomplete assessment for the underlying cause or inadequate treatment will lead to a persistent cough that appears to be unexplained.3 The most recent 2016 CHEST guideline on the treatment of adult RCC/UCC recommends that before diagnosis and before labeling the case as “difficult to treat,” providers should perform careful investigations of the potential causes of cough, as well as provide appropriate treatment and follow-up based on best-practice guidelines. In addition, the provider should confirm that the patient has been adherent to treatments.3 Patients with chronic cough should also undergo objective testing for bronchial hyperresponsive-ness and eosinophilic bronchitis or a therapeutic corticosteroid trial.3 Eosinophilic airway diseases will respond to corticosteroids, whereas noneosinophilic chronic cough will likely not.2
Pathophysiology of Refractory or Unexplained Cough
The exact mechanism(s) of RCC/UCC is still being investigated, but several mechanisms have been proposed. RCC/UCC may be due to an exaggerated cough reflex sensitivity to nontussigenic stimuli (ie, cough hypersensitivity syndrome).8 Recent advances in neuro-biology suggest that inflammation-induced injury to the neural pathways in the airway and the brain may lead to sensitization and chronic hypersensitivity syndrome.14
Overview of the Cough Reflex
Sensory nerve receptors to tussigenic stimuli have gated ion channels,2 which can be activated by a variety of triggers including acid, adenosine triphosphate (ATP), arachidonic-acid derivatives, bradykinin, and heat.2 Afferent fibers from airway cough receptors converge through the vagus nerves in the brainstem, and the nucleus tractus solitarius is connected to the central cough generator, which coordinates the efferent cough response.2,7
An enhanced cough reflex may result from increased sensitivity to any of the neuronal pathways involved in the cough response. These hypersensitivities may have resulted from initial cough triggers that are at one point treatable. However, the underlying causes of cough as well as the cough itself can lead to upper airway mucosal inflammation and tissue remodeling, resulting in an enhanced cough reflex (Figure).15 For example, upper respiratory tract infections are often followed by cough, which can lead to damage in the airway mucosa and inflammatory changes in the sensory nerves.15 In addition, common respiratory viruses, such as human rhinovirus, can upregulate the transient receptor potential (TRP) sensory nerve receptors. Repeated coughing activates inflammatory mechanisms through the release of chemical mediators, resulting in cough reflex hypersensitivity.15
A hypersensitivity reaction may be due to cough receptors in the periphery that have become sensitized and hyperresponsive or from altered central processing by the brainstem of cough signals received from the periphery (central sensitization).2,22
Peripheral Sensitization
Peripheral sensitization may occur in areas in which sensation is mediated by the vagus nerve, including the larynx, esophagus, pharynx, nasal cavity, and bronchi.15 Inflammatory mediators, such as prostaglandins and histamine, sensitize afferent nerve fiber endings (C fibers), resulting in excitation of the afferent nerves and a reduction of the cough threshold.15
Chronic cough is associated with airway injury or inflammation, both of which may release ATP into the airway.23 In both healthy individuals and patients with chronic cough, ATP inhalation triggers coughing.24 ATP stimulates vagal sensory neurons upon binding to the P2X purinoceptor 3 (P2X3) receptor,23 located predominantly on afferent neurons of sensory C fibers.24,25 ATP-induced action potentials in sensory C fibers can be inhibited by P2X3 antagonists,24 making P2X3 an important target in addressing peripheral sensory neuropathy in RCC/UCC.
In chronic cough, there is also increased expression of TRP vanilloid-1 (TRPV1) receptors on epithelial nerves in the airways.2,15 TRPV1 is the receptor for capsaicin and can also be activated by heat, acidity, arachidonic-acid derivatives, bradykinin, and ATP.2 TRP ion channels in airway sensory neurons mediate the transmission of nociceptive information to the brain.26 The activation of TRP ion channels, including TRPV1, and airway sensory nerves induces the cough reflex.26 In RCC/UCC, increased expression of TRPV1 has been observed, and the quantity of TRPV1-positive nerves is associated with cough sensitivity to inhaled capsaicin.26 Furthermore, TRPV1 inhibitors ameliorate the cough response induced by allergen challenge in a sensitized animal model.2
Central Sensitization
Central sensitization in chronic cough involves increased excitability in the central sensory pathways.15 Similarities exist between RCC/UCC and neuropathic pain, another condition with central sensitization. Features of neuropathic pain, including paresthesia, hyperalgesia, and allodynia, have corresponding features in RCC/UCC; namely, laryngeal paresthesia or hypersensitivity, hypertussia, and allotussia.15
RCC/UCC may also be due to reduced activation of cortical regions associated with voluntary cough suppression.27,28 In a study of 30 UK patients with RCC/UCC from a specialist cough clinic, the ability to suppress cough when challenged with nebulized capsaicin (a known tussive agent) was significantly lower in the patients with RCC/UCC compared with healthy subjects (P <.0001).27 Impaired ability to suppress cough points to dysfunction in central neural pathways in patients with RCC/UCC.27 In a study using functional magnetic resonance imaging during capsaicin inhalation among patients with chronic cough, investigators observed both central amplification of cough sensory inputs and reduced ability to suppress cough motor behavior.28
Current Treatment Approaches
In patients with chronic cough, if specific causes can be identified, treatment should be directed at the identified causes.9 If no cause can be identified, one strategy is to utilize sequential or concomitant empiric treatment to rule out other causes such as asthma, nonasthmatic eosinophilic bronchitis, gastroesophageal reflux disease (GERD), and upper airway cough syndrome.9 Inhaled corticosteroids are indicated only in adult patients with positive tests for bronchial hyperresponsiveness or eosinophilia, and proton pump inhibitors are indicated only in patients with GERD.3
The CHEST guideline recommends that if after excluding identifiable and treatable causes of cough, including lack of patient adherence, the chronic cough does not resolve, the patient is considered to have RCC/UCC or difficult-to-treat cough.3 The CHEST guideline recommends a therapeutic trial of multimodality speech pathology therapy.3 Some examples of speech pathology treatment include cough suppression swallow, cough control breathing, hydration, and avoidance of known irritants, in addition to patient counseling/education regarding motivation, adherence, and realistic goals.15,29
There are currently no approved agents for the treatment of chronic cough, but several agents have been used off-label. The CHEST guideline recommends a trial of gabapentin after a risk-benefit assessment and then another risk-benefit assessment at 6 months before continuing longer.3 This recommendation is based on results of a double-blind, placebo-controlled trial conducted by Ryan et al among patients in Australia with chronic cough lasting longer than 8 weeks.30 Patients were randomized to gabapentin titrated with a 6-day dose escalation (and reduction) schedule at the start (and end) of treatment to a maximum tolerable daily dose of 1800 mg (n = 32) or matching placebo (n = 30) for 10 weeks.30 Gabapentin significantly improved cough-specific quality of life (P = .004), as measured by the Leicester Cough Questionnaire (LCQ), and decreased cough frequency (P = .028) and self-reported cough severity visual analog scale (VAS) scores (P = .029).30 However, the benefits were not sustained after stopping treatment, and changes from baseline to after treatment discontinuation were not significant (LCQ score, P = .30; cough frequency, P = .88; cough severity VAS, P = .29).30 Blurred vision and disorientation or confusion were more common in patients receiving gabapentin.30 According to the CHEST guideline, the recommended starting dose of gabapentin is 300 mg once daily, with daily titration as tolerated up to a maximum of 1800 mg daily in 2 divided doses.3
Another centrally active agent, pregabalin, has been evaluated as an add-on to speech therapy. Vertigan et al conducted a placebo-controlled, double-blind trial in Australia and randomly assigned patients with RCC/UCC to pregabalin at a maximum dose of 300 mg daily or placebo for 12 treatment weeks (of the 14-week study period).31 Both groups received speech therapy. Pregabalin significantly improved LCQ scores (mean difference in LCQ = 3.5 [1.2]; 95% CI, 1.1-5.8 [P = .024])31 and reduced cough severity VAS (25.1 [7.1]; 95% CI, 10.6-39.6 [P = .002],31 but not cough frequency, compared with placebo.31 Cognitive changes, dizziness, blurred vision, and weight gain occurred more frequently in the pregabalin group.31
The efficacy of gabapentin, an agent used for seizures and neuropathic pain, in chronic cough support the notion that RCC/UCC may be due to aberrant neurophysiology.24 Gabapentin and pregabalin are centrally active and are mood-modulating therapies, which may explain their beneficial effect on quality of life and cough severity scores.7 It has been suggested that gabapentin and pregabalin lead to changes in mood and therefore improvement in perception of cough without true antitussive effect.7
Besides gabapentin and pregabalin, codeine, morphine, and amitriptyline have also been used as antitussives. The use of codeine is not fully supported by clinical trials. Most studies, although not performed in patients with RCC/UCC, do not show significant benefits compared with placebo.32-34 Although data from a single study with low-dose morphine indicate that it may have antitussive effects,35 safety concerns include respiratory depression, addiction, and drowsiness.24 The safety of long-term use of morphine in patients with RCC/UCC is unknown, and its use for cough is not recommended.24 To our knowledge, amitriptyline has been evaluated only with subjective rating of cough improvement and cough-specific quality of life, but not objective cough frequency among patients with RCC/UCC, so its effectiveness as an antitussive is unclear.36 Lastly, dextromethorphan, approved by the FDA in 1958 as an over-the-counter agent,37 has shown efficacy in reducing cough frequency in patients with acute cough, but its efficacy in chronic cough is unknown.38
Unmet Needs in Refractory or Unexplained Chronic Cough
Most of the currently available antitussives are centrally acting agents primarily used for neuropathy and pain. Although results from studies of these agents31,35,36 validate the notion that centrally acting mechanisms are potential therapeutic targets in RCC/UCC, the currently available agents were not developed for the treatment of cough and have limitations regarding their efficacy and safety.3 There is a high rate of treatment failure. In a case series of patients with sensory neuropathic cough lasting more than 8 weeks, approximately 40% of patients encountered a lack of treatment response to the initial neuromodulator used.39 In addition, over 50% of patients with chronic cough treated with available agents develop tachyphylaxis or dependence.13 Sedation is a concern with centrally active agents such as gabapentin, pregabalin, amitriptyline, and low-dose morphine.40 The last antitussive drug approved by the FDA, dextromethorphan, is indicated only for the management of acute cough.37 There are currently no FDA-approved agents for the treatment of chronic cough.40 Hence, there is a considerable unmet need for effective, safe, and nonsedating medications for the treatment of RCC and UCC.40
Recent Advances and Novel Agents in Chronic Cough
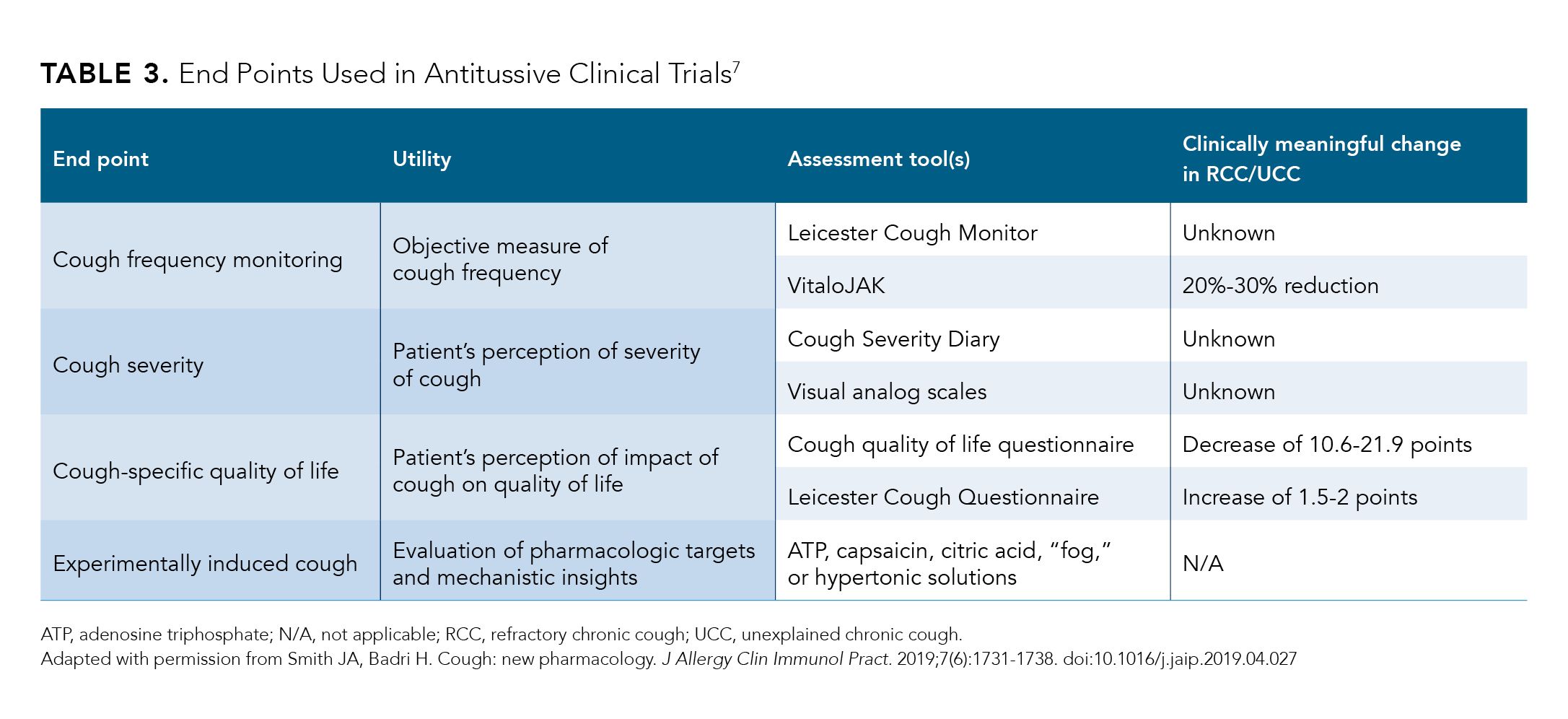
Several agents are under development, including 1 from Merck in a phase 3 trial for the treatment of chronic cough.7 End points used in clinical trials include cough frequency, cough severity, cough-specific quality of life, and experimentally evoked cough (Table 3).7 The ability to objectively quantify cough frequency using continuous acoustic cough monitoring technology has advanced the standards by which new antitussive agents are evaluated.7 Patient-reported outcomes, including cough-specific quality of life as assessed by the LCQ, can further confirm perceived improvements in cough symptoms and their effects on quality of life.7 Novel agents are under development for both the peripheral and central nervous system (CNS) neuronal targets of cough.7
Peripheral Neuronal Targets
Agents with peripheral neuronal targets may potentially avoid CNS adverse events (AEs) such as sedation.7 Therapy may also be delivered directly to the airways by inhalation, which may decrease systemic AEs.7
P2X3 receptor antagonists
As discussed in the pathophysiology of RCC/UCC, ATP is released during inflammation and binds to the purinergic P2X3 receptor.24 Hence, the P2X3 receptor is a target for drug development aiming at decreasing peripheral sensitization in chronic cough. Importantly, the P2X3 receptor has been detected only in afferent sensory C fibers and not in human brain tissues, making it an attractive target in treating peripheral sensory neuropathic features of RCC/UCC.24
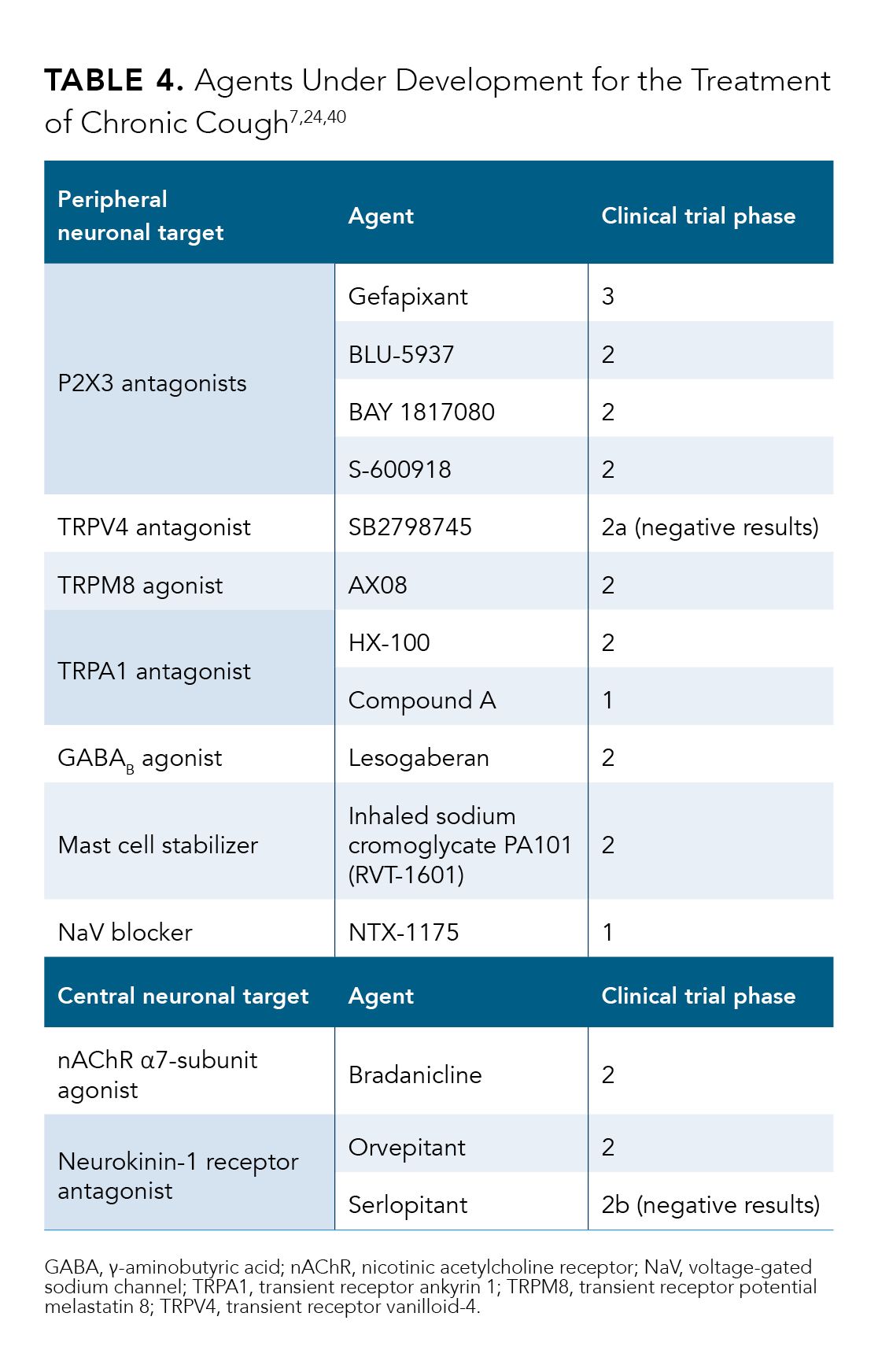
There are several P2X3 antagonists in various stages of development (Table 4).7,24,40 Gefapixant is a P2X3 antagonist being evaluated in phase 3 clinical trials, and preliminary results are available.41,42 Other P2X3 investiga-tional agents are in earlier phases of development.40
Gefapixant
Abdulqawi et al conducted a proof-of-concept, double-blind, placebo-controlled, crossover study and randomized 24 patients with RCC/UCC to gefapixant 600 mg twice daily followed by placebo twice a day or placebo twice daily followed by gefapixant 600 mg twice a day, for 2 weeks.43 After a 2-week washout period, patients received the alternative treatment.43 The primary end point, daytime objective cough frequency as assessed by an ambulatory cough recorder, decreased from a mean of 37 coughs per hour at baseline to approximately 11 coughs per hour after treatment with gefapixant and from 65 to about 44 coughs per hour with placebo (P = .0003).43 Secondary end points, including 24-hour cough frequency (P = .001), changes in daytime cough severity VAS scores (P = .003), urge to cough VAS scores (P = .035), and CQLQ scores (P = .018) also significantly improved with gefapixant compared with placebo.43 No serious AEs occurred during the study. Taste disturbance (dysgeusia) was reported by all patients and led to discon-tinuation in 6 patients.43 Taste disturbances associated with gefapixant were likely due to the high dose of gefapixant used in the study and the inhibition of P2X2/P2X3 hetero-meric receptors at this dose.23
Gefapixant’s effect on cough reflex sensitivity upon evoked tussive challenge was further evaluated in an additional study by Morice et al. In a phase 2, double-blind, crossover study, healthy volunteers (n = 12) and patients with chronic cough (n = 24) were randomized to gefapixant 100 mg (2 gefapixant 50-mg tablets) and placebo (2 matching placebo tablets) and were exposed to sequential challenges of inhaled ATP, citric acid, capsaicin, and distilled water at 1, 3, and 5 hours after dosing. Participants received the alternative treatment after a minimum 48-hour washout period. Cough reflex sensitivity was assessed by the lowest concentration of inhaled challenges to evoke at least 2 coughs (C2) and at least 5 coughs (C5). For the coprimary end points, the mean inhalant concentration (threshold) to induce C2 and C5 increased significantly by 4.7-fold (P = .0006) and 3.7-fold (P = .0067), respectively, upon ATP challenge in patients with chronic cough receiving gefapixant compared with placebo. The threshold for distilled water at C2 and C5 increased to a lesser extent but significantly by 1.4-fold and 1.3-fold, respectively (P < 0.05), in patients with chronic cough. There was no effect on citric acid or capsaicin challenge. Gefapixant also significantly reduced cough frequency (P = .008), cough severity VAS (P = .004), and urge-to-cough VAS (P = .002) in patients with chronic cough. The study demonstrated that there were at least 2 distinct sensory pathways, ATP and TRPV4 (activated by distilled water), evoking cough. There was a high rate of dysgeusia associated with gefapixant (about 67% of patients with chronic cough).44
The studies by Abdulqawi et al and Morice et al demonstrated the potential clinical application of gefapixant in patients with chronic cough, but the high frequency of dysgeusia prompted a lower dose to be evaluated in subsequent trials.45,46 Additional dose-ranging studies reported that gefapixant at a lower dose (30 or 50 mg twice daily) reduced taste disturbances but maintained its effectiveness on decreasing objective cough frequency and improving patient-reported outcomes.45,46 In a single research study that included 2 double-blind, randomized, placebo-controlled, crossover, dose-escalation studies, patients with RCC/UCC for at least 1 year were randomized to placebo or escalating dose levels of gefapixant (4 twice-daily dose levels of gefapixant [50, 100, 150, and 200 mg] in study 1 and 4 twice-daily dose levels [7.5, 15, 30, and 50 mg] in study 2) twice daily for 16 days.45 In study 1 (n = 29), the primary end point, 24-hour cough frequency at baseline and on day 4 of each dose, was significantly lower compared with placebo at all dose levels (50-200 mg daily; P = .05 for all analyses). In study 2 (n = 30), significant efficacy in 24-hour cough frequency was seen at a dose level of at least 30 mg (mean [± SD] 24-hour cough frequency vs placebo, 19.5 ± 17.6 vs 34.5 ± 30.8 at 30 mg [P = .05], and 20.8 ± 20.5 vs 37.3 ± 25.9 at 50 mg [P = .05]).45 Significant improvements versus placebo in secondary end points, including cough severity VAS (mean [± SD], 31.2 ± 23.3 vs 49.5 ± 24.7 [P = .05]) and cough severity diary (mean [± SD], 2.9 ± 1.9 vs 4.0 ± 1.7 [P = .05]), were also seen at the 30-mg dose level, as well as all higher dose levels.45 Quality of life as measured by LCQ at the highest dose level in both studies was significantly improved with gefapixant compared with placebo (200 mg in study 1, 15.4 ± 4.2 vs 12.3 ± 3.4 [P = .05] and 50 mg in study 2, 16.2 ± 4.1 vs 13.4 ± 3.9 [P = .05]).45 Taste disturbances were dose related and were reported in about 81% of patients at the highest doses (150 and 200 mg in study 1) and in approximately 47% and 53% at the 30-mg and 50-mg dose groups in study 2.45
In a phase 2b double-blind, placebo-controlled, parallel-group, dose-ranging study, patients with RCC/UCC lasting at least 1 year were randomized to placebo (n = 63) or oral gefapixant 7.5 mg (n = 64), 20 mg (n = 63), or 50 mg (n = 63) twice daily for 84 days.46 For the primary end point, gefapixant 50 mg twice daily showed a significant reduction, –37.0% (95% CI, –53.3 to –14.9; P = .0027), in objective awake cough frequency relative to placebo, and a reduction of 57.6% from baseline. Change in 24-hour objective cough frequency relative to placebo was –37.6 (95% CI, –53.1 to –16.9, P = .0014)%.46 Patient-reported outcomes (secondary end points), including cough severity VAS (P = .0108), cough severity diary total score (P = .0197), and LCQ score (P = .0028), also significantly improved compared with placebo at the 50-mg dose.46 Dysgeusia occurred in 48% of patients receiving gefapixant 50 mg twice daily.46
Preliminary results of 2 companion placebo-controlled, randomized, double-blind, international phase 3 trials, COUGH-1 (n = 732) and COUGH-2 (n = 1317), evaluating gefapixant have been reported.41,42,47 Enrolled patients had RCC/UCC for at least 1 year. In both studies, patients were randomized to either placebo, oral gefapixant 15 mg twice daily, or oral gefapixant 45 mg twice daily.42 In both studies, gefapixant 45 mg twice daily significantly reduced 24-hour cough frequency: –18.5% relative to placebo (95%CI –32.92 to –0.86; P = 0.041) at 12 weeks in COUGH-1, and –14.6% relative to placebo (95% CI –26.07 to –1.43; P = 0.031) at 24 weeks in COUGH-2.41 The 15-mg twice-daily dose did not meet the primary efficacy end point in either study.41
Investigation of other P2X3 antagonists (BAY 1817080, BLU-5937, and S-600918) is under way in phase 2 clinical trials (Table 4).7,24,40
Transient receptor potential receptors
There has been an emerging interest in the TRP ion channel family as possible therapeutic targets for airway diseases, including RCC/UCC.26 Several TRP channels are implicated in the cough reflex. Airway C-fiber sensory neurons express TRPV1 channels, through which heat, acidity, and capsaicin may evoke a cough.7 Two TRPV1 antagonists have been evaluated, but no effect on cough frequency was observed.7 TRP ankyrin 1 (TRPA1) recep-tors, another type of TRP channel, are activated upon exposure to irritant chemicals, such as cigarette smoke and perfumes, and lead to coughing in susceptible patients.7 An initial study evaluating TRPA1 antagonism reported negative results.7 Other drugs for this target are in early phases of investigation.7 Another TRP receptor, the TRP vanilloid-4 (TRPV4) receptor, plays a role in ATP release and subsequent P2X3 channel activation.7 TRPV4 antago-nism may allow blockade of P2X3 activation upstream, potentially avoiding the taste-related AEs. However, a proof-of-concept study evaluating the TRPV4 antagonist SB2798745 was stopped early due to a lack of efficacy in reducing cough frequency.7 Finally, TRP melastatin 8 (TRPM8) may mediate the cooling properties of menthol to reduce throat irritation. A pilot study of a TRPM8 agonist has been performed, but results are not yet available.7 Table 47,24,40 summarizes the compounds targeting the TRP family in various stages of development.
GABAB receptor agonists
The inhibitory neurotransmitter γ-aminobutyric acid (GABA), is widely distributed in both neuronal and non-neuronal systems.24 Lesogaberan is a peripherally acting GABAB receptor agonist originally developed for the treatment of GERD but has also shown antitussive poten-tial.24 A proof-of-concept study showed a nonsignificant 26% reduction in awake cough frequency compared with placebo and a significant improvement in capsaicin-induced cough.7 Lesogaberan is currently in phase 2 clinical development (Table 4).7,24,40
Sodium cromoglycate
Sodium cromoglycate is classified as a mast cell stabilizer, but its activity is thought to be more complex.24 Inhaled sodium cromoglycate has shown a possible antitussive effect in patients with lung cancer and patients with angiotensin-converting enzyme-induced cough.7 A recent study of high-dose nebulized cromoglycate suggests that it may be effective as an antitussive in patients with idiopathic pulmonary fibrosis but not in RCC/ UCC.7 A larger dose-ranging phase 2b trial is ongoing to evaluate the effect of nebulized cromoglycate on 24-hour cough frequency among patients with idiopathic pulmonary fibrosis.7
Sodium channel blockade
Nebulized lidocaine has been used by some clinicians to treat RCC/UCC. Lidocaine blocks voltage-gated sodium channels. Recent studies of 2 novel sodium channel blockers were stopped due to lack of efficacy.48 One agent in this class is in phase 1 development (Table 4).7,24,40
Central Neuronal Targets
One advantage of targeting the CNS is the potential to modulate numerous neuronal pathways as they converge in the brainstem or higher centers. Central agents under development in chronic cough include neurokinin-1 (NK1) receptor antagonists and nicotinic acetylcholine receptor (nAChR) α7-subunit agonists.7
Neurokinin-1 receptor antagonism
NK1 receptors and their ligand, substance P, play a role in the CNS in cough responses.7 Two NK1 antagonists, orvepitant and serlopitant, are in phase 2 studies in patients with RCC/UCC.7 Results from the phase 2 study (VOLCANO-1; EU Clinical Trials Register No. 2014-003947-3) for orve-pitant have been published.49 In 13 patients with RCC/UCC, orvepitant at 30 mg daily for 4 weeks significantly decreased objective daytime cough frequency.49 Coughs per hour decreased by a mean of 18.9 (26%; 95% CI, 9.6-28.3; P < .001).49 VOLCANO-2 (NCT02993822), a randomized, multicenter, controlled, dose-ranging study, is ongoing (Table 4).7,24,40
nAChR α7-subunit agonism
Nicotine inhibits capsaicin-induced cough, and the nAChR α7-subunit may be responsible for nicotine’s antitussive effect.24 A phase 2 trial of bradanicline, a selective agonist of nAChR α7-subunit in patients with refractory chronic cough, has recently been completed (Table 4).7,24,40
Conclusions
Cough is one of the most common reasons for medical office visits in the United States.1 Cough may be more than a symptom of underlying conditions; up to 10% of patients with chronic cough have chronic cough of unexplained origin.3,4 Chronic cough markedly impairs patients’ quality of life, work, and social functions and leads to heavy health care resource utilization.3,5,6 Several pathophysiologic mechanisms may underlie RCC/UCC, including hyper-sensitivity of neurons triggering the cough reflex.14 There are currently no approved therapies for the treatment of chronic cough. Novel targets for antitussive therapies are in various phases of clinical development. Phase 3 clinical studies have shown promise for oral gefapixant 45 mg twice daily, and a significant reduction in 24-hour cough frequency compared with placebo at 24 weeks was observed among patients with RCC/UCC.41,42 Therapy for the management of RCC/UCC is currently an unmet need, and new options on the horizon may improve patients’ quality of life while decreasing the health care resource utilization associated with this condition. ◆
REFERENCES
1. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed October 6, 2020. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371(9621):1364-1374. doi:10.1016/s0140-6736(08)60595-4
3. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44. doi:10.1378/chest.15-1496
4. Barbee RA, Halonen M, Kaltenborn WT, Burrows B. A longitudinal study of respira-tory symptoms in a community population sample. Correlations with smoking, allergen skin-test reactivity, and serum IgE. Chest. 1991;99(1):20-26. doi:10.1378/chest.99.1.20
5. Zeiger RS, Schatz M, Butler RK, et al. Burden of specialist-diagnosed chronic cough in adults. J Allergy Clin Immunol Pract. 2020;8(5):1645-1657.e7.
doi:10.1016/j.jaip.2020.01.054
6. French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657-1661. doi:10.1001/archinte.158.15.1657
7. Smith JA, Badri H. Cough: new pharmacology. J Allergy Clin Immunol Pract. 2019;7(6):1731-1738. doi:10.1016/j.jaip.2019.04.027
8. Al-Hajjaj MS. Management of chronic unexplained cough. Ann Thorac Med. 2017;12(1):1-2. doi:10.4103/1817-1737.197761
9. Michaudet C, Malaty J. Chronic cough: evaluation and management. Am Fam Physician. 2017;96(9):575-580.
10. Irwin RS, French CL, Chang AB, Altman KW; CHEST Expert Cough Panel. Clas-sification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153(1):196-209. doi:10.1016/j. chest.2017.10.016
11. Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagno-
sis and treatment of chronic cough in adults and children. Eur Respir J.
2020;55(1):1901136. doi:10.1183/13993003.01136-2019
12. Morice AH, McGarvey L, Pavord I, British Thoracic Society Cough Guideline Group. Recommendations for the management of cough in adults. Thorax. 2006;61(suppl 1):i1-24. doi:10.1136/thx.2006.065144
13. Bowen AJ, Huang TL, Nowacki AS, et al. Tachyphylaxis and dependence in pharmacotherapy for unexplained chronic cough. Otolaryngol Head Neck Surg. 2018;159(4):705-711. doi:10.1177/0194599818788062
14. McGarvey L, Gibson PG. What is chronic cough? Terminology. J Allergy Clin Immunol Pract. 2019;7(6):1711-1714. doi:10.1016/j.jaip.2019.04.012
15. Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ.
2015;351:h5590. doi:10.1136/bmj.h5590
16. French CT, Fletcher KE, Irwin RS. A comparison of gender differences in health-related quality of life in acute and chronic coughers. Chest. 2005;127(6):1991-1998. doi:10.1378/chest.127.6.1991
17. Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis. 2010;4(1):49-55. doi:10.1177/1753465809358249
18. Hulme K, Dogan S, Parker SM, Deary V. ‘Chronic cough, cause unknown’: a quali-tative study of patient perspectives of chronic refractory cough. J Health Psychol. 2019;24(6):707-716. doi:10.1177/1359105316684204
19. Kuzniar TJ, Morgenthaler TI, Afessa B, Lim KG. Chronic cough from the patient’s perspective. Mayo Clin Proc. 2007;82(1):56-60. doi:10.4065/82.1.56
20. Sinha A, Porter T, Wilson A. The use of online health forums by patients with chronic cough: qualitative study. J Med Internet Res. 2018;20(1):e19. doi: 10.2196/jmir.7975
21. Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms
among patients with chronic cough. Chest. 2006;130(6):1839-1843. doi:0.1378/chest.130.6.1839
22. Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersen-sitivity syndrome in respiratory medicine. Eur Respir J. 2014;44(5):1132-1148. doi:10.1183/09031936.00218613
23. Muccino D, Green S. Update on the clinical development of gefapixant, a P2X3 re-ceptor antagonist for the treatment of refractory chronic cough. Pulm Pharmacol Ther. 2019;56:75-78. doi:10.1016/j.pupt.2019.03.006
24. Song WJ, Chung KF. Pharmacotherapeutic options for chronic refractory cough. Expert Opin Pharmacother. 2020;21(11):1345-1358. doi:10.1080/14656566.2020. 1751816
25. Ford AP, Undem BJ. The therapeutic promise of ATP antagonism at P2X3 recep-tors in respiratory and urological disorders. Front Cell Neurosci. 2013;7:267.
doi:10.3389/fncel.2013.00267
26. Millqvist E. TRPV1 and TRPM8 in treatment of chronic cough. Pharmaceuticals (Basel). 2016;9(3):45. doi:10.3390/ph9030045
27. Cho PSP, Fletcher HV, Turner RD, Jolley CJ, Birring SS. Impaired cough suppression in chronic refractory cough. Eur Respir J. 2019;53(5):1802203.
doi:10.1183/13993003.02203-2018
28. Ando A, Smallwood D, McMahon M, Irving, L, Mazzone, SB, Farrell, MJ. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax. 2016;71(4):323-329. doi:10.1136/thoraxjnl-2015-207425
29. Vertigan AE, Haines J, Slovarp L. An update on speech pathology management of chronic refractory cough. J Allergy Clin Immunol Pract. 2019;7(6):1756-1761. doi:10.1016/j.jaip.2019.03.030
30. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a ran-domised, double-blind, placebo-controlled trial. The Lancet. 2012;380(9853):1583-1589. doi:10.1016/s0140-6736(12)60776-4
31. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combi-nation therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648. doi:10.1378/chest.15-1271
32. Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 1997;49(10):1045-1049.
doi:10.1111/j.2042-7158.1997.tb06039.x
33. Hutchings HA, Eccles R. The opioid agonist codeine and antagonist naltrexone do not affect voluntary suppression of capsaicin induced cough in healthy subjects. Eur Respir J. 1994;7(4):715-719. doi:10.1183/09031936.94.07040715
34. Smith J, Owen E, Earis J, Woodcock A. Effect of codeine on objective measure-ment of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117(4):831-835. doi:10.1016/j.jaci.2005.09.055
35. Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175(4):312-315. doi:10.1164/rccm.200607-892OC
36. Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neurop-athy. Laryngoscope. 2006;116(12):2108-2112. doi:10.1097/01.mlg.0000244377.60334.e3
37. Dicpinigaitis PV, Morice AH, Birring SS, et al. Antitussive drugs—past, present, and future. Pharmacol Rev. 2014;66(2):468-512. doi:10.1124/pr.111.005116
38. Morice A, Kardos P. Comprehensive evidence-based review on European antitus-sives. BMJ Open Respir Res. 2016;3(1):e000137. doi:10.1136/bmjresp-2016-000137
39. Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuro-pathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816. doi:10.7717/peerj.816
40. Dicpinigaitis PV, McGarvey LP, Canning BJ. P2X3-receptor antagonists as potential antitussives: summary of current clinical trials in chronic cough. Lung. 2020;198(4):609-616. doi:10.1007/s00408-020-00377-8
41. Merck’s gefapixant (45 mg twice daily) significantly decreased cough frequency compared to placebo at week 12 and 24 in patients with refractory or unexplained chronic cough. News release. Merck. September 9, 2020. Accessed October 22, 2020. https://www.merck.com/news/mercks-gefapixant-45-mg-twice-daily-significantly-decreased-cough-frequency-compared-to-placebo-at-week-12-and-24-in-patients-with-refractory-or-unexplained-chronic-cough/
42. Merck announces top-line results from phase 3 trials evaluating gefapixant, an investigational treatment for refractory or unexplained chronic cough. News release. Merck. March 17, 2020. Accessed October 22, 2020. https://www.merck. com/news/merck-announces-top-line-results-from-phase-3-trials-evaluating-gefapixant-an-investigational-treatment-for-refractory-or-unexplained-chronic-cough/
43. Abdulqawi R, Dockry R, Holt K, et al. P2X3 receptor antagonist (AF-219) in refrac-tory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198-1205. doi:10.1016/s0140-6736(14)61255-1
44. Morice AH, Kitt MM, Ford AP, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1):13993003.00439-2019. doi:10.1183/13993003.00439-2019
45. Smith JA, Kitt MM, Butera P, et al. Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J. 2020;55(3):1901615.
doi:10.1183/13993003.01615-2019
46. Smith JA, Kitt MM, Morice AH, et al; Protocol 012 Investigators. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. 2020;8(8):775-785. doi:10.1016/s2213-2600(19)30471-0
47. A dose escalation study of bradanicline in refractory chronic cough. ClinicalTrials. gov. Updated June 20, 2019. Accessed October 22, 2020. https://clinicaltrials.gov/ct2/show/results/NCT03622216
48. Pipeline. Nocion Therapeutics. Accessed October 22, 2020. https://www.nociontx. com/#pipeline
49. Smith J, Allman D, Badri H, et al. The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1). Chest. 157(1):111-118. doi:10.1016/j.chest.2019.08.001
Innovations in Chronic Cough Treatments
A Q&A With Alyn Morice, MD, FRCP
The American Journal of Managed Care® (AJMC®): How do you distinguish chronic cough as a stand-alone disease versus a symptom of another type of condition?
MORICE: Well, it’s usually fairly obvious because the patient says they’ve been coughing for a long time—months and years. My record is 66 years [of] chronic cough. These patients suffer enormously for years on end and have a large amount of health care utilization. They are going to see an average, in my clinic, of 6 doctors beforehand and have had innumerable tests done. Nobody can find the answer because they don’t recognize the disease of chronic cough.
AJMC®: How is a chronic cough measured? What is your process for making a diagnosis of chronic cough?
MORICE: It’s very simple. I get the patient to score the cough out of 10. The patient will say, “What do you mean, doctor?” I say, “Well, just give me a number out of 10 for your cough, where 10 is worst, and zero is no cough.” It’s a very simple marker, and it’s recommended in the European Respiratory Society [ERS] guidelines on chronic cough, which [were] published this year. That’s the clinical way of doing it in a clinic. You don’t need any fancy tests or anything.
How to make a diagnosis? Well, it usually involves trying to exclude most other illnesses. A normal chest x-ray will exclude almost all the things like cancer and so on. We recommend in ERS guidelines, a simple chest x-ray, and if that’s normal, then you’ve got to rely on the history, and the history of a person with chronic cough has a very characteristic story. If you ask the right questions, you’ll get the right answers. We here have developed a questionnaire called Hull Airway Reflux Questionnaire [HARQ], which targets the diagnostic questions that you need to ask, such as hoarseness and problem[s] with the voice, cough with meals, cough while lying down and bending over, [and] cough on rising in the morning. These are all very characteristic of this particular syndrome. Indeed, in the 2000 patients who were in the Merck gefapixant phase 3 clinical trials, COUGH-1 and COUGH-2, they used the HARQ at entry, and normal people score 4 out of 70. The upper limit of normal is 14. The average in these 2000 patients was 40. I use this HARQ question[naire] every day. It’s available on our website for free [at] ISSC.info. I administer it to the patients, and then it also guides me when I’m talking to the patient in the clinic to ask the right set of questions, because if they answer zero to a question about hoarseness for the voice, I don’t bother asking about the voice. It’s a very useful tool, and it’s being widely used now, [as well as] in Europe.
AJMC®: What are some common comorbidities associated
with chronic cough?
MORICE: The [one] common is irritable bowel syndrome, but there are things like obesity. It is predominantly a first-world problem because, as we all know, the population is getting more and more obese, and the incidence of chronic cough appears to be increasing in parallel with that. All of this points to the fact that it’s a problem with the upper [gastrointestinal] tract. These people mainly suffer from a form of reflux, but it’s not acid reflux or [gastroesophageal reflux disease]. It is a nonacid reflux, which doctors, particularly gastroenterologists, find very difficult to understand. It goes up and irritates the nerves in the throat, and that leads to cough hypersensitivity. The characteristic of these patients is that they have supersensitive nerves in their throat. This leads to the tickle, and they get exposed to external things like change in temperature, strong smells, bleaches, [and] perfumes, and that triggers the coughing attack because the nerves are supersensitive.
AJMC®: At the European Respiratory Society International Congress 2020, you gave a presentation titled “Chronic Cough—Symptom or Disease?” Can you give us some highlights of that presentation?
MORICE: I use as the backbone the ERS guidelines, which came out this year and are therefore the most up-to-date. Indeed, it’s the only international guideline, really. The Asian Pacific Society of Respirology, [and] the Chinese Thoracic Society have endorsed it, so it’s an international guideline. If you look at [the] COUGH-1 and COUGH-2 studies, they are very typical of the chronic cough patient. It’s predominantly middle-aged [women] who get cough, and that’s because women have an increased cough sensitivity compared to men. The typical patient is a [woman] in her 50s or 60s who has had the cough, in my clinic, for approximately 6 years on average. The mean number of years in COUGH-1 was 11.8 and in COUGH-2 was 10.7. The trials are of a very typical patient. They were predominantly acting with 75% women who presented to the international centers, in COUGH-1 and COUGH-2. I’ve already mentioned that they all scored very highly on the HARQ. That’s a diagnostic test, and we can now objectively record cough. What we did in…this study was to use a cough monitor…at various points [and] count the number of coughs over 24 hours. That was the primary end point of the study…to show a reduction in the 24-hour cough counts.
The results were really quite spectacular. [At] the 45-mg dose of gefapixant, there was a reduction in coughing by about 60%. What I’m telling people is that if you give this drug to patients with this typical syndrome, [for example,] middle-aged [women] with chronic cough, 60% of them will walk out in effect cured by this treatment.
In the trial, there was quite a large placebo effect. We do see [the] placebo effect in cough studies, just as you see it in migraine studies, for example. Some people have said, “Oh, it’s a placebo response.” Well, in fact, I think I have an idea why there is such a large placebo response in these particular trials, but I’m investigating that at the moment. In the trial, the 45-mg dose, as I [said], treated about two-thirds of patients with almost complete resolution of their cough, and [in] my own personal experience, because I must have given it now to about 200 patients, it works almost immediately. We’ve had people who’ve been picked up by their husbands in clinical trials, maybe a bit late, and the patient has remarked, in the hour they’re waiting for the husband to come, “Oh, my cough’s gone, doctor.” People come in tears saying, “Doctor, this is the first time I’ve stopped coughing in 20 years.” It [is] the most rewarding thing as a physician to treat these patients because they are so desperate. It’s a hidden disease because the patient can’t go out and they can’t go to the church or the synagogue, they can’t go to restaurants, they can’t go to the cinema because they get heckled and accused, and particularly in [coronavirus disease 2019] times, their lives are a complete misery. They become basically recluse. If they’ve been to 5 doctors and 5 doctors have told [them that] there’s nothing wrong with them — they only [have] a cough; what are they worried—about they just give up. There’s a lot of hidden misery out there with these patients, which can be easily treated with this class of drugs.
AJMC®: How can awareness of chronic cough as a disease be improved among physicians?
MORICE: The heavy lifting is going to be the education because, particularly in the [United] States, there is a great deal of ignorance of the disease of chronic cough. In Europe, we’ve got far more cough centers than you have in the [United] States, and we have the ERS, [which is] an organization [that] has actually endorsed chronic cough as the disease. The [American Thoracic Society] or the American College of Chest Physicians, although they do recognize chronic cough, they don’t recognize it as a separate disease. They think it’s part of asthma or COPD [chronic obstructive pulmonary disease], which it definitely is not.
If you’re a patient and go along to see a practitioner, who is not au fait with the fact that chronic cough is a disease, and you say, “I’ve been coughing now for the last year, doctor,” they will try to fit you into the boxes of the diseases that they know. So overwhelming[ly], [the] majority of young people or younger people are diagnosed as [patients with] asthma. If you’ve ever smoked a cigarette at all, in your life, you get diagnosed with COPD, but patients actually have a chronic cough as a disease rather than either of those diseases, which are fundamentally different in many aspects.
I think the best way to promote awareness of chronic cough for a drug company such as Merck [should] be direct patient advertising. If the patient goes along and say[s], “Hi, here, there’s a treatment for my cough,” the doctor won’t know about chronic cough and will be actually forced by the patient to go and learn more about it. There is a barrier for the poor patients: the ignorance of the physician.
AJMC®: Can you describe the mechanism of action and efficacy of currently available chronic cough treatments?
MORICE: OK, so there are 4 drugs in development. Merck and the Canadian company Bellus [Health] both have drugs that are looking very good. We’ve just [been] doing a trial with that. We’re about to publish the phase 2 [study] from Bayer, and again, [they’re] extremely promising results, [with] their cough reduction, improvement in quality of life, and all the rest of it. The final one is [the] Japanese company called Shionogi.
All 4 of these drugs are what are called P2X3 antagonists. P2X3 is an ion channel that’s located on nerves, particularly the vagus nerve, which is the one that’s in the throat, and the vagus nerve transmits the irritation. As I said earlier, the nerve is hypersensitive. We can show in the laboratory that people…who have a chronic [cough] respond more to passive stimuli like capsaicin or citric acid. So, these people have hypersensitive nerves and cause the hypersensitivity, [which] is the release of ATP [adenosine triphosphate] by the cells lining the throat or lining the airways.
This ATP acts as what’s called an alarmin. It goes onto this P2X3 receptor on the nerves and opens the channel. It depolarizes the nerve, giving rise to the tickle and
the irritation. So, P2X3 antagonists block for that channel opening and prevent the hypersensitivity of the nerve.
AJMC®: Can you describe the overall clinical and economic burden associated with chronic cough?
MORICE: Well, there’s been quite a lot of work done on that recently. The figures are coming out that the patients suffer enormously, [and] as I said, in terms of quality of life, that has an enormous impact on their economic and financial abilities. I have patients who work in call centers, for example, and they can’t do it because they’re coughing all the time. People have had to leave their teaching jobs because they can’t work with coughing and going into meetings, and in the middle of a presentation, having a coughing bout and losing their voice [is a challenge]. It’s a major factor in people, and people retire early. [In] the personal sense, it’s got a large economic burden, but also, because the patient does try to repeatedly go back to the doctor, there are lots of consultations. Because the doctor doesn’t understand the cough, they do [a lot] of unnecessary investigations. There are bronchoscopies, methacholine challenge, and a whole range of different tests [that] are done looking for the mysterious cause of this cough. Whereas, if they asked the right questions, it would be…obvious what’s wrong.
It’s [a] great time to be in cough because this is a completely new therapeutic area. I think we’re sort of going out into an empty prairie area at the moment. We just got to the first field, and basically, I think the drugs will also work on breathlessness. In the community out there, there are a whole lot of people who are seeing severe asthma specialists, and the reason they’ve got “severe asthma” is because they haven’t got asthma at all. They’ve got this chronic cough, which the doctor can’t get better with asthma drugs. Misdiagnosis—COPD and asthma, as I said—is going to be a major hurdle to get them out of the clutches of these asthma doctors into [treatment by] someone who knows what we’re talking about. I mean, the amount of money that’s spent on asthma treatment is absolutely astronomic[al], and yet, these drugs will actually, I think, help a large amount of people with “severe asthma” because the doctor doesn’t recognize that it’s not asthma, it’s cough. ◆
Understanding Chronic Cough
A Q&A With Albert A. Rizzo, MD, FACP, FCCP
The American Journal of Managed Care® (AJMC®): How would you describe the current awareness of chronic cough among US physicians?
RIZZO: I think most physicians in the United States realize that cough is a very common symptom that presents to their office. Unfortunately, chronic cough tends to be something that not only presents [to] their office but also gives them some degree of frustration at times because many of these patients don’t seem to respond the way we would like them to. I think it’s been recommended that probably 12% of the people that have cough probably have cough that really can’t be explained well enough or treated well enough. For this reason, patients tend to come back with current symptoms. I think physicians realize that there are multiple, but still a limited number of, causes for chronic cough. I think many physicians realize that a fair amount of literature and guidelines have been put in place with regard [to] how to approach patients with chronic cough, but it is a population of patients that require a little bit of extra time and thought when they do present to the office. I think physicians are very aware, [and] I think it is something that they would like to be able to handle a little bit better.
AJMC®: What is your definition and process for making a diagnosis of chronic cough? How does it differ from other types of cough? Is there a test for chronic cough?
RIZZO: By definition, usually, chronic cough is defined as a cough that’s lasting [for] at least 8 weeks or longer. As far as making the diagnosis of chronic cough, the key thing is [a] really pretty detailed history and physical, because most of the time, and probably up to maybe 80% to 90% of the time, that history and physical will often give clues as to what is the most common reason for this patient to have a chronic cough. Most of these patients fall into several categories, the first one being cough explained by what’s called postnasal [drip] syndrome or upper airway cough syndrome. These are things called sinusitis, allergic rhinitis, something in the upper airway; it’s triggered by either allergies, irritants, or anatomical problems with drainage. The other big area falls into the category of pulmonary diseases, undiagnosed asthma, early diagnosis of [chronic obstructive pulmonary disease], bronchiectasis, [and] interstitial fibrosis; so those are all specific lung conditions that could be a reason for the chronic cough. A third big category is that of gastroesophageal reflux disease or laryngeal reflux disease, related to acid in the esophagus or reflux up to the larynx that leads to irritation of the larynx.
I think those 3 categories are pretty well recognized as causes for much of the chronic cough that can be explained. Then you have that smaller percentage of people who have the refractory or unexplained chronic cough. As far as a test for chronic cough, most of the testing has to do with determining whether or not those 3 conditions I mentioned, either upper airway syndromes, pulmonary syndromes, or gastroesophageal reflux syndromes, exist. The tests include, usually, imaging, which could include CT [computerized tomography] scans or x-ray [of] the sinuses, chest x-ray, CT scan of the chest, [and] studies looking at esophageal reflux, whether it’s endoscopy or pH probe monitoring. All of those tests help determine if 1 of the 3 known causes of chronic cough [is] present. They can’t rule them out, but they can certainly help rule them in if those tests come back consistent with a reason for the chronic cough. For example, pulmonary function tests may be very normal in somebody with chronic cough, but if there’s a stimulation test, or what’s called a bronchoprovocation test with methacholine performed, it may show that the airways are more sensitive, and this could be a diagnosis of asthma that’s not been recognized. That certainly can be a reason for recurring cough, especially when the right triggers are there. I think triggers are the important thing because when you’re doing this history and physical, you’re really trying to determine, is there a trigger for this cough? Is there an activity? Is there a time of day? Are there certain air-quality issues, time of year, humidity, [or] seasonality? Are there things that can be identified as bringing the cough on? An important trigger would be to realize that the cough has been brought on by the initiation of a[n] antihypertensive drug like lisinopril.
We know that the ACE [angiotensin-converting enzyme] inhibitors…are a common reason for chronic cough. This can occur possibly [in] up to 15% to 20% of the patients who go on an ACE inhibitor. Recognizing [that] the cough coincides with the initiation of the drug is important, but having said that, people can be on this drug for months to years prior to developing the cough related to the ACE inhibitor. That, again, is an important historical point to bring out with regard to medications the patient is on, whether or not that is a trigger. Are there different types of cough? Certainly, I think the nature of the cough can be helpful—dry cough versus moist cough. Certainly, a cough productive of sputum that looks … yellow or green can often suggest the presence of an infection. This may be coming from the airways, it may be coming from the sinuses, but the cough itself is usually triggered by 1 of the 3 mechanisms I mentioned earlier: upper airway, pulmonary, or [gastrointestinal] reflux.
AJMC®: How does the physician-and-patient relationship play a role in diagnosis?
RIZZO: Knowledge is the biggest tool...being able to [know how to] talk to your physician [when] explaining your symptoms and understanding why certain tests may need to be done or questioning why certain tests aren’t being done. We—again, the [American Lung Association]—are trying to make sure patients have the information they need, and it’s in a manner that helps them deal with their interaction with their physicians. I would recommend certainly looking at the lung.org website under “cough” [as a search term]. That’s the main thing, and arm yourself with information about the situation and be as honest and thorough as you can when you’re talking to your doctor about your symptoms.
AJMC®: What are the current challenges for patients with chronic cough in the United States?
RIZZO: I think the biggest challenge is that the patients keep going to their physician and are not really getting the kind of relief that they want in order to improve what has turned into a quality-of-life issue. Many times, these patients are dealing with coughs that are prolonged, they’re loud. They’re disturbing to not only the patient but also those around them, [and it] becomes embarrassing for the patient. I think it is a matter of quality of life, and that is the biggest challenge [in] dealing with a cough that’s just not being addressed appropriately.
The other challenge is that many times, patients will come away from a physician’s office with a list of procedures or tests that need to be done in order to come up with some answers. That may or may not be the first time they’re given that list of tests to do. Many times these patients, because they’re not getting the kind of satisfaction they need or the result of resolution of the symptoms, will seek different opinions, not only from primary care physicians; they may start to seek out specialists, and this may fall into the category of allergists or pulmonologists, gastroesophageal gastroenterologists, [or] ENT [ear, nose, and throat] specialists. There’s a host of specialists, and again, it becomes challenging for the patient to find one that’s appropriate for them to see, based on their insurance coverage, [as] there are often costly co-pays for many of these subspecialist evaluations. It becomes almost a financial challenge at the same time that it is a challenge to deal with a disruptive symptom that again, disrupts not only them but also their family and people around them. They become almost isolated because they’re afraid to go to gatherings, where they may be pointed out as the person who keeps coughing during the symphony [or] during church; all those things tend to weigh on the patient as a challenge.
AJMC®: What is the American Lung Association doing to drive awareness in the area of chronic cough as its own disease state?
RIZZO: Like a lot of things we do at the American Lung Association, we break our mission down into usually 3 components: education, research, and advocacy. Now, in chronic cough education, and maybe a better term is awareness, that’s an important part of what we have on our website [and] is available for individuals to peruse at lung. org. The main thing that our website will help them with is identify[ing] what a chronic cough is...What are some of the causes? Also, there is a questionnaire on that website for the patient to fill out so they start to realize that maybe they are in that category of a patient who, despite all the testing that’s been done and all the specialists they’ve seen, may have what’s called a refractory uncontrolled chronic cough, not related to some disease that would otherwise be apparent. That could be one outcome. The other outcome might be that…an individual’s had a chronic cough and did not realize that there are multiple reasons for it, and they haven’t really started down the road of seeking a specialist to help determine the [cause of] cough. That may become something they feel like they have to live with. They may have tried over-the-counter medications for a number of weeks to months, with seeing some improvement but not really getting the resolution they need. So, I think the website raises awareness [and] gives them some tools to start identifying where are they with regard to their journey in this diagnosis. Who should they be seeing based on what they have done already? What might be some next steps?
Other parts that we do, like I mentioned, involve aware-ness and education. We do have some research studies looking at whether or not certain chemicals are helpful in the cough. A common source [that] patients look at sometimes [is] zinc...for cough resolution.
I think the other part of research is to realize that many of the over-the-counter medications that have been out there represent probably a $7 billion market [and] have never really been tried-and-true with regard to clinical trials showing that there is evidence that they work. This includes everything from sprays to maybe liquids or pills. All of these things are on the market and available over the counter. Patients try multiple ones in order to get some relief—again, getting partial relief from time to time, and that could be a placebo effect, rather than necessarily a true medication effect based on studies that really haven’t been shown to be helpful. The other part with regard toadvocacy around this area has to do with making sure patients have the proper coverage from their insurance company to get the tests that are recommended to be done. There also is concern that we might want to make sure that patients realize that cough by itself may be an entity that needs to be looked at as a specific disease by itself and [is] not always a symptom related to some other diseases. These are all things that we’re looking at to try to improve access to care for patients.
AJMC®: Can you describe the clinical and economic burden associated with chronic cough for physicians, patients, and policy makers?
RIZZO: As I alluded to earlier, many times these patients are on a merry-go-round with regard to seeking different opinions from physicians [and] doing tests probably multiple times over the course of months to years when they’re not really getting the answers they want. The cost of care [is] surely reflected in the fact that there is an unmet need to control these symptoms and deal with the issues that affect their quality [of] life. So, anything that can be done to move along science to find an appropriate treatment would be helpful, and again, I think it is a matter of making sure that insurers understand that this is a diagnosis that is not easily handled by necessarily
1 or 2 specialists and often requires a team approach. There are very few, but there are some specialty clinics across the country, cough clinics, that put together multidisciplinary teams, including doctors—as I mentioned earlier, allergist; pulmonologists; ear, nose, and throat physicians; [and] speech therapists, all in a unified manner to help deal with this combination of chronic cough that can’t be explained easily by the other diseases I mentioned—but those are few and far between. Those may be things that will be helpful as we get a better knowledge of what chronic cough is, and certainly when there is a potential treatment out there for chronic cough.
AJMC®: Can you elaborate on the unmet needs for real therapies to differentiate between cough and cold? What treatments currently in development do you find promising?
RIZZO: As I mentioned, there are lot of unmet need[s] from the standpoint of really sorting out what works and what doesn’t work with all [to] these over-the-counter medications that people spend more and more money on. I think the realization is that up to 90% of these patients can receive an appropriate diagnosis and be put on treatment for their upper airway syndromes, pulmonary syndromes, or [gastrointestinal] symptoms if they see the right doctors and have the right test[s] done. But in order to meet the needs of those remaining patients with the chronic cough, there does need to be further investigation of what’s triggering these coughs. The cough reflux is thought to be a combination of something [that] irritates the airway, and this airway is either hypersensitive or hyperresponsive, more than it should be, and sends a message to the brain that then makes the patient cough with the muscles of the diaphragm and the chest muscles. That hypersensitivity might be able to be approached from a neuroreceptor standpoint, with a drug that affects that hypersensitivity or hyperresponsiveness in the airway.
There are drugs on the market now that affect neuroreceptors. For example, gabapentin is used to treat chronic cough. It does have adverse effects that have to be watched for. There are potential treatments in the pipeline to look at that [GABA] receptor and whether or not that receptor can be affected. There are several large pharmaceutical companies that are looking at this very actively. The specific enzyme, I think it is called [a] P2X3 receptor, is being targeted by companies such as Merck and Bellus. With their drugs that have been in development over the last several years, none are on the market, [and] several are working their way through the phase trials to reach FDA approval. But again, we’re going to have to wait and see whether these drugs are not only effective enough to make a difference but also whether they’re tolerable enough and safe enough to make a difference when they are ultimately approved. ◆
