- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Addressing Healthcare Disparities and Managed Care Considerations With Continuous Glucose Monitoring
To claim CE credit for this activity, please visit https://www.pharmacytimes.org/courses/analyzing-the-value-of-continuous-glucose-monitoring-in-diabetes-care-and-overcoming-barriers-through-expanded-pharmacy-access
What Are Social Determinants of Health, Health Disparities, and Health Equity?
The settings where people are born, live, work, and grow old can profoundly influence personal health, overall well-being, and quality of life (QOL).1 These conditions, known as social determinants of health (SDOH), encompass economic security, healthcare access and quality, education access and quality, neighborhood and environment, and interpersonal relationships in the community. While SDOH can have a positive or negative impact on health, social risk factors are social conditions that are associated with poor health. For instance, household income is a SDOH, whereas household income below the poverty level may be a social risk factor for poor health.2
Thus, dissimilarities in SDOH between certain groups may lead to health disparities.3 According to Healthy People 2020, a health disparity is “a particular type of health difference that is closely linked with social, economic, and/or environmental disadvantage.” Historically, groups of people have encountered systemic discrimination and obstacles affecting health based on age, disabilities, ethnicity, faith, geography, gender identity, psychiatric illness, race, sex, sexual orientation, and socioeconomic status (SES).
Addressing health disparities in the United States is not new to the public health agenda. The Healthy People 2020 goals, for instance, included achieving health equity or the opportunity for all individuals to achieve their highest level of health.4 Released by the US Department of Health and Human Services every 10 years, the guidance notes that achieving equity “requires valuing everyone equally, with focused and ongoing societal efforts to address avoidable inequalities, historical and contemporary injustices, and the elimination of health and healthcare disparities.”3 The Healthy People 2030 goal for diabetes continues a focus on reducing disparities and decreasing the incidence, complications, and diabetes-related mortality for all persons with diabetes.5
Health Disparities in Diabetes Prevalence and Outcomes
Approximately 37 million people in the United States (11.3% of the population) have diabetes, of which an estimated 8.5 million adults are undiagnosed.6 An additional 96 million adults in the United States have prediabetes.6 Diabetes-related complications such as stroke, chronic kidney disease, blindness, and amputation can cause substantial morbidity, contributing to annual diabetes direct medical costs of approximately $237 billion, $90 billion of which are related to reduced productivity (2017).7 Diabetes was also the eighth leading cause of death in the United States in 2020 after adjusting for age.8 While a common chronic condition, there are significant differences in the prevalence of diagnosed diabetes and outcomes in adults by race, ethnicity, SES, education, and age in the United States.9
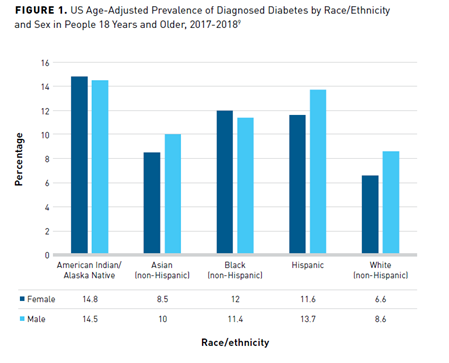
The highest rates of diabetes occur in Native American/Alaska Native individuals, followed by Hispanic, non-Hispanic Black, Asian, and non-Hispanic White individuals (Figure 19). Rates further vary among Asian (non-Hispanic) groups, with Asian Indian and Filipino individuals having a higher prevalence of diagnosed diabetes (12.6% and 10.4%, respectively) versus Chinese (5.6%) individuals. Hispanic individuals of Mexican (14.4%) and Puerto Rican (12.4%) descent have higher rates of diagnosed diabetes than those of Central/South American (8.3%) or Cuban (6.5%) heritage.9
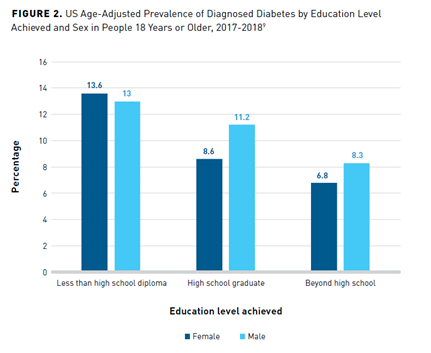
As indicated by the level of education achieved, SES also profoundly affects the prevalence of diabetes.9 Adults who do not graduate from high school have a higher age-adjusted prevalence of diagnosed diabetes than those with more than a high school education (Figure 29).
In addition to prevalence, diabetes quality of care, disease control, and outcomes are worse in minorities and individuals with low SES.10-12 For instance, Black Medicare beneficiaries with diabetes have worse glycemic control, are more likely to develop end-stage renal disease, and are significantly less likely to receive retinal eye exams than White patients.13-15
Health Disparities in Access to Continuous Glucose Monitoring
Indications for Use of Continuous Glucose Monitoring
The primary goal of diabetes management is to reduce patient risk of diabetes-related comorbidities and complications through education, lifestyle management, and drug therapy, with a primary target of improving glycemic control.16 Data from randomized controlled trials, observational, and real-world studies have shown a role for CGM in diabetes care by demonstrating that CGM use leads to A1C lowering, improved time in range (TIR), and reduction in hypoglycemia.17
Based on the evidence, the American Diabetes Association (ADA) Standards of Medical Care in Diabetes–2022, Diabetes Technology guidelines provide recommendation to providers regarding the use of CGM. This guidance recommends that providers offer real-time CGM (rtCGM) or intermittently scanned CGM (isCGM) to persons with diabetes requiring insulin who can (or their caregivers can) safely use it.17 This includes:
- Adults with diabetes on multiple daily injections or using an insulin pump
- Adults on basal insulin
- Youth with diabetes on multiple daily injections or using an insulin pump
- Persons with diabetes who are pregnant in addition to pre- and postprandial blood glucose monitoring (BGM)
The 2021 American Association of Clinical Endocrinology Advanced Technology CGM guideline recommendation aligns with the ADA’s for patients on insulin and pregnant women but adds that CGM should be considered for all individuals with frequent/severe hypoglycemia, nocturnal hypoglycemia, and hypoglycemic unawareness, regardless of insulin use.18 The 2016 Endocrine Society guidelines recommend the near-daily use of rtCGM in adult persons with type 1 diabetes (T1D) and short-term, intermittent rtCGM use in persons with type 2 diabetes (T2D) not on prandial insulin who have A1C levels greater than or equal to 7% and who are capable and willing to use rtCGM.19
Disparities in Use of Continuous Glucose Monitoring
Despite aligned and clear guidelines for use, disparities in access to CGM technology exist in historically marginalized groups within specific racial/ethnic populations and lower SES groups.20 Several studies have examined racial disparities in CGM use in children and young adults with diabetes. One such study by Chen et al was a secondary data analysis of a randomized controlled trial conducted at the Joslin Diabetes Center and Texas Children’s Hospital.21 This study identified disparities in CGM access based on race/ethnicity as well as other sociodemographic, diabetes-specific, and psychosocial characteristics in 301 participants aged 13 to 17 years with T1D. During the study period, 6% of patients used CGM technology at baseline, and 21% started during the study period.21
CGM was higher in non-Hispanic White patients than patients of another race/ethnicity (88% vs 70%; P = .009). In addition, CGM users had a statistically significantly greater likelihood of coming from a family with an annual income of $150,000 or more (44% vs 23%; P = .0001), with private health insurance (95% vs 82%; P = .005), or a parent with at least a college education (81% vs 64%; P = .004). Relative to disease burden and management, CGM users had a higher frequency of BGM (5.2 ± 1.9 vs 4.3 ± 1.9; P = .0002). However, there were no statistically significant differences in youth-reported family involvement or disease burden and no parent-reported differences in youth adherence, diabetes burden, and diabetes-specific family conflict between users and nonusers.21
A retrospective chart review by Lai and colleagues of children younger than 17 years with T1D attending the Children’s Hospital of Philadelphia between January 1, 2015, and December 13, 2018, also found racial/ethnic disparities in CGM initiation and continued use.22 Of 1509 eligible participants, 73% were non-Hispanic White, 18% were non-Hispanic Black, and 8% were Hispanic. Of the 726 (48%) who started CGM, more non-Hispanic White children (54%) started CGM than non-Hispanic Black (31%) and Hispanic children (33% [P <.0001]). Non-Hispanic White children were more likely to start CGM versus non-Hispanic Black (odds ratio [OR], 2.7; 95% CI, 1.9-3.8) or Hispanic children (OR, 2.4; 95% CI, 1.5-3.9). Of the children who started CGM before age 17, significantly fewer non-Hispanic Black patients (61%) continued CGM use at 1 year (Hispanic: 85%, non-Hispanic White: 86%; P <.001). Non-Hispanic White children were more likely to continue CGM use after 1 year than non-Hispanic Black children (OR, 4.1; 95% CI, 2.1-7.7). However, the odds of continuing CGM use at 1 year did not differ between non-Hispanic White and Hispanic children (OR, 1.1; 95% CI, 0.3-3.8).22
A third, multicenter, cross-sectional study of 300 young adults (100 were non-Hispanic White, 97 were non-Hispanic Black, and 103 were Hispanic) with T1D aged 18 to 25 years examined the effect of demographics, clinical variables, SES, diabetes self-management, and healthcare factors on CGM use among racial/ethnic groups.23 Fifty-five percent of the participants were women, with an average age of 20 years and a mean diabetes duration of 10 years. The average A1C was 10.5% for non-Hispanic Black participants, 8.6% for Hispanic participants, and 8.1% for non-Hispanic White participants. A greater percentage of non-Hispanic Black and Hispanic participants had lower and similar SES indicators, including income less than $50,000 (both 74%), than non-Hispanic White participants (33%) and a higher neighborhood poverty percentage (non-Hispanic Black: 22%, Hispanic: 19%) than non-Hispanic White (9%) participants. CGM use was the lowest in non-Hispanic Black (28%) participants, followed by Hispanic (37%) and non-Hispanic White (71%) participants. Disparities remained for non-Hispanic Black patients when adjusting for demographics, SES, diabetes self-management, and healthcare factors (non-Hispanic Black: 31%, Hispanic: 58%, non-Hispanic White: 53%).23
Reasons for Disparities in Access to Continuous Glucose Monitoring
Implicit Bias
Implicit bias happens when providers unconsciously base medical decisions on stereotypes and prejudice. As a result, racial and ethnic minorities may be less likely to receive diagnostic tests, preventive care, and procedures to manage their health, and may contribute to disparities in healthcare access and outcomes.24
The Gatekeeper study of pediatric providers of T1D care examined if implicit bias against public insurance, an indicator of low SES, influenced CGM and insulin pump recommendations.25 The researchers identified implicit bias through the Diabetes Provider Implicit Bias tool, a hypothetical case and ranking exercise of patient factors important to prescribing CGM or an insulin pump. After viewing 1 of 2 identical case vignettes, with public or private insurance being the only difference, providers were asked if they would offer diabetes technology.25
Thirty-nine providers completed the Diabetes Provider Implicit Bias tool (physicians: n = 19, nurse practitioners: n = 12, certified diabetes educators: n = 8). The average age of providers was approximately 44 years, around 80% were non-Hispanic White, and roughly 90% were women. Implicit bias was identified in approximately 85% of healthcare providers (HCPs). After adjusting for age, sex, race/ethnicity, provider role, percent of public insurance served, and workplace location, the study found that the number of years in practice was positively associated with the likelihood of implicit bias (adjusted OR, 1.47; 95% CI, 1.02-2.13; P = .007). The patient factor that HCPs considered most important for recommending CGM use was family preference, followed by insurance type, BGM frequency, A1C, child’s age, and family income. Providers ranked race/ethnicity as the least important consideration for recommending CGM use.25
Insurance
Other studies suggest insurance may play a role in disparities in CGM and other diabetes technology access and outcomes. One of the studies examined data from 4895 participants in the T1D Exchange from 2015 to 2016 to find indirect and direct relationships between access to diabetes technology, insurance coverage features, SES, and adverse outcomes, including hypoglycemia and diabetic ketoacidosis (DKA).26 Cost and insurance barriers to an insulin pump or CGM were somewhat important for approximately 27% of participants, with roughly 46% reported to be burdened by any health-related costs and/or insurance barrier. Around 41% of participants reported insurance covers some costs of CGM, approximately 20% covers all costs, roughly 13% does not cover any costs, and around 27% were unknown. In a structural equation model, better insurance coverage was significantly associated with CGM use (β = 1.21, SE = 0.14; P <.0001) as was a higher SES (β = 1.52, SE = 0.12; P <.0001). A significant direct and protective relationship between CGM and adverse outcomes was also observed (β = -0.23, SE = 0.06; P = .0002). More generous insurance coverage and SES demonstrated indirect associations with fewer adverse outcomes due to increased technology access (β = -0.40, SE = 0.09; P <.0001 and β = -0.33, SE = 0.09; P = .0002, respectively).26
Other reports have identified disparities by insurance. Medicaid enrollees are known to experience more barriers to healthcare, lower quality of diabetes management, poorer diabetes control, and experience more acute and long-term complications from diabetes.27 However, a recent ADA paper also reported that Medicaid recipients with diabetes using insulin were 2 to 5 times less likely than those with a commercial insurance plan to use a CGM device.28 Notably, race/ethnicity continued to be a driver of disparities in CGM use in the Medicaid population, with Black and Hispanic enrollees having disproportionately lower rates of CGM use than White Medicaid recipients.27
Managed Care Considerations
Real-World and Economic Evidence
Real-world outcomes data and economic evaluations contribute to the evidence managed care decision makers can evaluate in establishing coverage and reimbursement policies for health technology, including CGM. This related body of evidence includes a 12-month, real-world prospective, observational cohort study of patients using isCGM.29 This study was conducted in 1913 participants with T1D in Belgium and assessed the association between isCGM use and QOL, as well as glycemic control. QOL remained stable from baseline to 12 months as measured by the 36-item Short Form Survey, Problem Areas in Diabetes scale, and Hypoglycemia Fear Survey–Worry. However, Diabetes Treatment Satisfaction Questionnaire status scores improved significantly (28.0 [95% CI, 26.1; 29.9] vs 30.4 [95% CI, 28.5; 32.2]; P <.0001).29
Perceived frequency of hyperglycemia and hypoglycemia scores were higher when using isCGM, suggesting greater awareness of glycemic control. Meanwhile, the cohort mean A1C was 7.8% at baseline, 7.7% at 6 months, and 7.8% at 12 months, and admission rates for severe hypoglycemia and/or DKA declined from 3.3% before the study to 2.2% at 12 months (P = .031). In addition, fewer participants required help from third parties for hypoglycemia (14.6% vs 7.8%; P <.0001), experienced hypoglycemic comas (2.7% vs 1.1%; P = .001), and missed work (5.8% vs 2.9%; P <.0001) from baseline to 12 months. The percentage of time in level 1 or level 2 hypoglycemia also improved from the first 2 weeks of the study (level 1: 5.1% [4.3-6.0], level 2: 4.0% [2.6-5.3]) to 12 months (level 1: 4.5% [3.6-5.3], level 2: 3.5% [2.1-4.9]). TIR decreased slightly overall from the first 2 weeks to 12 months (-0.9% [-1.5, -0.3]); however, TIR was dependent on frequency of scans. For those who scanned 7 to 10 times a day, TIR was stable, and it increased 1.3% (0.2-2.4) in those who scanned more than 10 times a day. Skin reactions were common, occurring in 11% of participants, but only 1% discontinued isCGM use.29
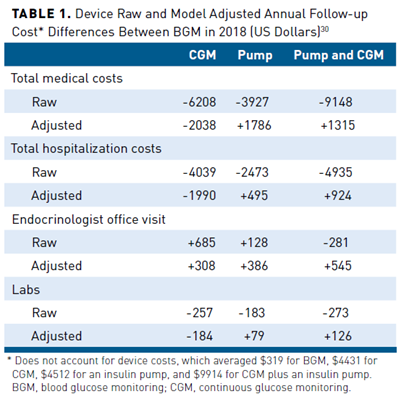
A 12-month, retrospective, observational study analyzed cost and utilization based on insurance claims data for 8235 participants with T1D or T2D using BGM (n = 5155), CGM (n = 966), an insulin pump (n = 1579), or CGM with an insulin pump (n = 535).30 Costs were from a US payer perspective and reported in 2018 US dollars. The unadjusted annual total medical costs were lower for CGM, insulin pump, and CGM plus insulin pump groups at baseline and 1-year follow-up than BGM. After multivariable adjustment, just the CGM group realized savings relative to BGM (Table 130). Adjusted total cost difference between CGM and BGM favored CGM for hospitalization and laboratory testing but not for office visits. The cost savings in Table 130 did not account for device costs, which were, on average, $319 for BGM, $4431 for CGM, $4512 for an insulin pump, and $9914 for CGM plus an insulin pump. Overall, healthcare utilization event rates favored CGM, an insulin pump, and CGM plus an insulin pump over BGM. For the CGM group, there were lower rates for emergency department visits, hospitalizations, and laboratory testing but not office visits (Table 230).
The DIAMOND trial was a randomized trial of rtCGM versus BGM in persons with T1D and an A1C of 7.5% or more using multiple insulin injections. Authors then conducted within-trial (6 months) and lifetime economic analyses from a societal perspective to examine the cost-effectiveness of rtCGM versus BGM in the DIAMOND population.31 Efficacy inputs from DIAMOND for rtCGM versus BGM, respectively, included reduction in A1C of -0.99% ± 0.77 versus -0.39% ± 0.70 (P <.01) and reduction in the daily rate of non-severe hypoglycemia events of -0.12 ± 0.29 versus -0.06 ± 0.27 (P = .02). Costs included direct and indirect costs in 2015 US dollars. Direct costs included medication, clinical care from trial personnel, healthcare services, CGM use, and test strip use. Indirect costs encompassed decreased work productivity and time devoted to diabetes care.31
In the within-trial analyses, mean total costs were higher in the rtCGM group ($11,032; n = 102) compared with the BGM group ($7236; n = 53), with the difference driven mainly by the cost of the rtCGM device despite lower use of daily test strips (change of -0.5 ± 1.5 vs 0.1 ± 1.5; P = .04). However, the mean utilities (CGM: 0.92 ± 0.12 vs BGM: 0.91 ± 0.10; P = .78) and quality-adjusted life-years (QALYs) were similar (CGM: 0.46 ± 0.06 vs BGM: 0.46 ± 0.05). Thus, rtCGM was dominated by BGM in the short-term analyses.31
In the lifetime analysis, costs for rtCGM showed incrementally higher total costs versus BGM ($55,208), but also a gain in QALY of 0.54 QALYs over BGM. Thus, the base-case incremental cost-effectiveness ratio was $98,108 per QALY (95% CI, $90,298 to $105,144 per QALY). Simulating a real-world situation by extending sensor use from 7 to 10 days reduced the incremental cost-effectiveness ratio to $33,459 per QALY.31
Recent Continuous Glucose Monitoring Eligibility Updates
The effective management of diabetes is a priority for the Centers for Medicare & Medicaid Services (CMS) given the high prevalence of diabetes in the Medicare population.32 CMS required patients to perform a minimum of 4 BGM tests per day for CGM initiation in the past but eliminated this requirement in July 2021. Thus, CMS coverage policies for CGM are now more aligned with ADA and Endocrine Society standards of care that recommend individualized testing frequency per patient need and glycemic goals.16,19,33 In addition, CMS updated the type of insulin for CGM coverage to include both inhaled and injectable insulin.34 CMS currently reimburses CGM devices and related supplies for beneficiaries meeting all of the following 5 coverage criteria:
- Having a diagnosis of diabetes
- Being treated with 3 or more daily insulin administrations or an insulin pump
- Who need to adjust insulin regimen based on BGM or CGM results frequently
- Who have had an in-person visit with the treating provider within the previous 6 months, who determines the beneficiary meets criteria 1, 2, and 3
- Who have had an in-person visit with the treating provider every 6 months after initiation, who assesses the beneficiary’s adherence to CGM and diabetes treatment plan
Coverage Expansion From Durable Medical Equipment to the Pharmacy: Implications and Considerations
The benefit from which CGM is covered may also impact patient access. CMS continues to reimburse CGM devices and related supplies through durable medical equipment (DME) benefits for those who meet all coverage criteria.34 However, CGM coverage through pharmacy benefits is becoming more common. For instance, a Center for Health Care Strategies report on Medicaid fee-for-service (FFS) access to CGM found that of the 41 states (including the District of Columbia) with published CGM criteria, 20 cover CGM devices under DME, while 21 cover CGM devices under the pharmacy benefit.27
Noted advantages of CGM coverage through the pharmacy benefit include the convenience to patients of being able to obtain the CGM device and supplies from their pharmacy in the same manner as medications and faster time to product availability. Whether these benefits translate to difference in the length of time from CGM prescription to initiation was examined in a retrospective cohort study of 271 individuals receiving diabetes care at an adult endocrinology clinic.35 Overall, 208 (78%) of those prescribed CGM initiated use during the study period. People who were younger, with private insurance, and who received certified diabetes education were more likely to initiate CGM successfully, while participants who were Black or Hispanic were less likely. Of those initiating CGM, 58% with CGM access through a pharmacy benefit initiated CGM in less than 30 days versus 2% of those accessing CGM through the DME benefit. While time to CGM initiation was significantly less when covered under a pharmacy benefit, it averaged over 3 months (78 days); this duration extended to 5 months when covered through a DME benefit (152 days; P <.0001).35
There are other advantages for payers in transitioning CGM coverage from DME to the pharmacy benefit, including the integration of CGM into formularies and preferred drug lists (PDL). Relative to Medicaid, states may include CGM devices on their PDL when covered through the pharmacy benefit. Although coverage benefit expansion from DME to the pharmacy may provide certain advantages for Medicaid members, Medicaid plans can apply utilization management tools to CGM, such as restrictive prior authorization criteria with burdensome documentation requirements and monthly prescription requirements for CGM supplies.27
Despite the possibility of such restrictions, the Center for Health Care Strategies report recommended that FFS Medicaid plans transition CGM coverage from a DME benefit to a pharmacy benefit, citing improved patient convenience. It also advocated for including both rtCGM with predictive alerts and isCGM with real-time alerts on the PDL to provide the best CGM option to fit the patient’s glycemic needs. The report also stated that manufacturer rebates might offset CGM costs and budgetary impact, making them more cost-effective.27 Thus, the analysis of data to demonstrate the value of CGM coverage may encourage states to remove restrictions and expand coverage to increase access.
Cost and Insurance Coverage Barriers
Results of an internet-based survey of adults with T1D or parents of children with T1D performed by the T1D Exchange provided insights as to why patients do not initiate CGM.36 About 55% indicated CGM was too expensive and approximately 40% stated it was not covered by insurance. Additionally, 52% of adult past users indicated that CGM was stopped due to noncoverage by insurance and 45% responded it was too expensive.36 In another survey study with 1503 adult participants with T1D, the cost of supplies (≈61%), the cost of the device (≈57%), and insurance (≈57%) were listed as barriers to CGM use.37
A report examining Medicaid FFS CGM criteria sheds light on the restrictive requirements for coverage that patients may face, which included27:
- Requiring prescribers to be endocrinologists or to have an endocrinologist consultation before prescribing
- Requiring regular visits with an HCP
- Providing CGM coverage solely for children
- Covering CGM solely in recipients with T1D
- Requiring the recipient to have at least one or more of the following:
- A minimum frequency of BGM testing
- A minimum of 3 injections of insulin per day
- Insulin pump therapy
- Hypoglycemic episodes, nocturnal hypoglycemia, refractory postprandial hypoglycemia, or hypoglycemic unawareness
- Recurrent DKA
A paper discussing current CGM eligibility criteria identified similar CGM eligibility criteria as barriers to care. Some private and governmental insurance plans also limit access based on a specified BGM frequency, the frequency of insulin dosage adjustment, the requirement of 3 or more daily insulin injections, an in-person provider assessment and monitoring of CGM use, and extensive documentation requirements to obtain CGM coverage.38
Processes/Strategies to Optimize Continuous Glucose Monitoring Data
Population Health Data Around Glucose Metrics
Chronic diseases place a financial burden on payers and patients.39 This includes diabetes, which, as mentioned previously, is associated with $237 billion in direct medical costs a year. To balance cost and quality goals, health plans may enter into a value-based contract (VBC) with providers. VBCs financially reward HCPs through incentive payments for the quality of care they give.40 VBCs are based on predetermined quality performance measures. Payers similarly enter into VBCs with manufacturers of medications and other health technologies. In this context, VBCs are contractual agreements in which the payment terms are tied to agreed-upon clinical circumstances, patient outcomes, or measures.41 Further, product coverage is often tied to a VBC between manufacturers and insurance payers and thereby affect patient access to medications and technology.42 Therefore, instead of focusing solely on the patient care or product volume, VBCs consider healthcare service and product value and outcomes to optimize resource use and improve population outcomes.42
Population health management is an important tool in managing VBCs that focuses on improving the health of a defined population, enhancing the quality of patient care, and reducing costs. The goal of population health management is to deliver the “right care to the right patient at the right time.”43 To achieve this goal, providers use data and analytics to differentiate risk and tailor interventions to optimize care and outcomes. It involves using data from multiple sources, such as claims data, medical records, and laboratory data, to identify the patients with the greatest need and deliver the most appropriate care.
CGM data can facilitate providers’ diabetes-related population health management efforts by allowing for more timely identification and management of poor glucose control. The utility of CGM data in population health management was recently detailed in a study assessing the impact of an algorithm and dashboard based on passively uploaded CGM data.44 The evidence-based algorithm was designed to identify children with new-onset T1D using CGM who would benefit from more intense (weekly) provider review via telemedicine. Compared with the control group, patients who were identified and received provider review had an 8.7% (95% CI, 0.6-16.9) improvement in TIR and a higher likelihood of experiencing a 5% improvement in TIR (70-180 mg/dL) during the following week (random effects model OR, 1.29; 95% CI, 1.02-1.64; P = .03; fixed effects model OR, 1.34; 95% CI, 1.05-1.71; P = .02).44
Potential Role of Continuous Glucose Monitoring Data in Quality Measures
Given the focus on quality and value, diabetes-related VBCs can be designed to meet the interests of patients, providers, payers, and manufacturers to reward care that improves patient care and health outcomes. However, currently, diabetes quality measures related to glycemic control and incorporated into VBCs are based on A1C, which is a delayed and imprecise measure of glucose control. In addition, A1C is an intermediary outcome that has relevance to diabetes control but may be less important to patients.
This discordance was observed in a modified Delphi Method survey study designed to identify outcome measures for T2D for VBCs related to diabetes medications that are most relevant to key stakeholders, including patients, endocrinologists, primary care physicians, payers, pharmacy benefit managers, and pharmaceutical company representatives.45 The 2 most important outcomes identified were reducing heart attack risk (84%) and decreasing A1C levels (68%). However, there was statistically significant discordance in the importance of some diabetes outcomes measures between patient and non-patient stakeholders. Reducing A1C was more important to non-patient stakeholders (37.5% vs 82.3%, respectively; P = .03), and reducing the risk of diabetes-related kidney disease was more meaningful to patients (50.0% vs 11.8%, respectively; P = .03).45
Thus, quality measures that better reflect outcomes of most interest to all stakeholders are warranted, and CGM data could help move towards that goal. Studies indicate that TIR is associated with the risk of microvascular complications while also correlating well with A1C.46,47 Monitoring time above and below range is helpful to adjust treatment to prevent hypoglycemia, hyperglycemia, and hospitalizations.
Given this evidence, the ADA 2022 Standards of Care suggest the use of 14-day CGM data of TIR as well as the glucose management indicator (GMI), a proxy for A1C, as alternate measures of glucose control in clinical decision making.17 The Diabetes Leadership Council in a 2020 Consensus Statement extended this recommendation to the population level, stating that, “Value-based insurance design in diabetes will fall short if payers and providers emphasize A1C but neglect TIR, reducing hypoglycemia, cardiovascular and renal protection, behavioral health, improved QOL, and other measures that people with diabetes value.”48 Additionally, the Center for Health Care Strategies’ report suggested that states with VBCs could consider adopting TIR as a quality measure and develop incentives for providers and insurance plans to increase access to CGM technology for better diabetes management.27
Transitioning to the use of CGM data in diabetes quality measures will require development and adoption of measures by quality assurance and measurement entities such as the National Committee for Quality Assurance (NCQA). Thus, it is notable that a recent NCQA white paper outlined recommendations for diabetes quality measures based on CGM data. Consistent with the positions outlined above, the NCQA white paper recommends the use of GMI as an alternative to A1C and the assessment of the proportion of patients with a 5% improvement in TIR, which has been associated with a reduction in microvascular complications.49 Thus, this NCQA white paper represents an important milestone in the evolution of diabetes quality assessment.
Finally, there is recognition that quality measures should consider social risk factors and address health disparities. Starting in 2021, the NCQA began to include race and ethnicity stratification in the Healthcare Effectiveness Data and Information Set dataset. Such stratification will encourage plans to collect complete and accurate race/ethnicity data. It will also provide insight into disparities in care and outcomes.50
Including more detailed glycemic control data from CGM devices in patient care and quality metrics while also stratifying quality measures on SDOH has potential to facilitate equitable access to CGM. Well-defined clinical CGM metrics like TIR and GMI could also be incorporated in VBCs and, when stratified by SDOH, may facilitate plan efforts to reduce disparities. VBCs may, therefore, serve as a catalyst for the generation of real-world data to bolster stakeholder support and reduce the financial risk associated with the equitable coverage and adoption of CGM technology.42
Conclusions
Substantial disparities in access to CGM technology exist among people of color and lower SES groups. Although Medicare CGM eligibility criteria have become less restrictive, cost and insurance requirements associated with CGM technology remain significant barriers to patient access, which in turn may exacerbate disparities. However, providing CGM coverage through the pharmacy benefit may help to increase patient convenience and access to the technology. In addition, the utility of CGM in diabetes management may be optimized by efforts to integrate CGM data into electronic health records using CGM data in population health management analytics. Implementing VBCs targeting CGM-based quality metrics may optimize glycemic control and improve diabetes outcomes. Real-world data generated by VBCs may increase stakeholder confidence and alleviate financial risk concerns to facilitate equitable and appropriate access to CGM technology.
Author affiliation: Carrie McAdam-Marx, PhD, RPh, is a Professor, Department of Pharmacy Practice and Science, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE
Funding source: This activity is supported by an educational grant from Abbott Diabetes Care Inc.
Author disclosure: Dr McAdam-Marx has no relevant financial relationships with commercial interests to disclose.
Authorship information: Analysis and interpretation of data; concept and design; critical revision of the manuscript for important intellectual content; drafting of the manuscript; supervision.
Address correspondence to: carrie.mcadammarx@unmc.edu
Medical writing and editorial support provided by: Lori A. Uildriks, PharmD
REFERENCES
- Social determinants of health. Healthy People 2030, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Accessed May 24, 2022. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
- Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q.2019;97(2):407-419. doi: 10.1111/1468-0009.12390
- Disparities. Healthy People 2020, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Accessed May 24, 2022. www.healthypeople.gov/2020/about/foundation-health-measures/Disparities
- Healthy People. Centers for Disease Control and Prevention, National Center for Health Statistics. Reviewed March 9, 2022. Accessed May 24, 2022. www.cdc.gov/nchs/healthy_people/index.htm
- Diabetes. Healthy People 2020, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Accessed May 24, 2022. https://health.gov/healthypeople/objectives-and-data/browse-objectives/diabetes
- National diabetes statistics report: estimates of diabetes and its burden in the United States. Centers for Disease Control and Prevention. Reviewed January 18, 2022. Accessed May 24, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
- Centers for Disease Control and Prevention. Diabetes report card 2019. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2021. Accessed May 24, 2022. www.cdc.gov/diabetes/pdfs/library/Diabetes-Report-Card-2019-508.pdf
- Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020. NCHS Data Brief, No. 427. National Center for Health Statistics, Centers for Disease Control and Prevention. December 2021. Accessed May 24, 2022. www.cdc.gov/nchs/products/databriefs/db427.htm
- Centers for Disease Control and Prevention. National diabetes statistics report, 2020; 2020. Accessed May 24, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Margolis DJ, Hoffstad O, Nafash J, et al. Location, location, location: geographic clustering of lower-extremity amputation among Medicare beneficiaries with diabetes. Diabetes Care. 2011;34(11):2363-2367. doi: 10.2337/dc11-0807
- Joseph JM, Johnson PJ, Wholey DR, Frederick ML. Assessing diabetes care disparities with ambulatory care quality measures. Health Serv Res. 2015;50(4):1250-1264. doi: 10.1111/1475-6773.12277
- Meneghini L. Ethnic disparities in diabetes care: myth or reality? Curr Opin Endocrinol Diabetes Obes. 2008;15(2):128-134. doi: 10.1097/MED.0b013e3282f5dbb8
- Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 2005;353(7):692-700. doi: 10.1056/NEJMsa051207
- Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in Medicare managed care. JAMA. 2002;287(10):1288-1294. doi: 10.1001/jama.287.10.1288
- Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol. 2007;18(4):1299-1306. doi: 10.1681/ASN.2006050524
- Draznin B, Aroda VR, Bakris G, et al; American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83-S96. doi: 10.2337/dc22-S006
- Draznin B, Aroda VR, Bakris G, et al; American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S97-S112. doi: 10.2337/dc22-S007
- Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505-537. doi: 10.1016/j.eprac.2021.04.008
- Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology–continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11):3922-3937. doi: 10.1210/jc.2016-2534
- Isaacs D, Bellini NJ, Biba U, Cai A, Close KL. Health care disparities in use of continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S3):S81-S87. doi: 10.1089/dia.2021.0268
- Chen CW, Tinsley LJ, Volkening LK, Anderson BJ, Laffel LM. Observed characteristics associated with diabetes device use among teens with type 1 diabetes. J Diabetes Sci Technol. 2021;19322968211050069. doi: 10.1177/19322968211050069
- Lai CW, Lipman TH, Willi SM, Hawkes CP. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2021;44(1):255-257. doi: 10.2337/dc20-1663
- Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306-313. doi: 10.1089/dia.2020.0338
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi: 10.1186/s12910-017-0179-8
- Addala A, Hanes S, Naranjo D, Maahs DM, Hood KK. Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the United States: findings from the Gatekeeper study. J Diabetes Sci Technol. 2021;15(5):1027-1033. doi: 10.1177/19322968211006476
- Everett EM, Wisk LE. Relationships between socioeconomic status, insurance coverage for diabetes technology and adverse health in patients with type 1 diabetes. J Diabetes Sci Technol. 2021;19322968211050649. doi: 10.1177/19322968211050649
- Howe G, Chavis J. Expanding Medicaid access to continuous glucose monitors. Center for Health Care Strategies. Published January 2022. Accessed May 24, 2022. www.chcs.org/media/Expanding-Medicaid-Access-to-Continuous-Glucose-Monitors_011222.pdf
- Health equity and diabetes technology: a study of access to continuous glucose monitors by payer and race executive summary. American Diabetes Association. Accessed May 24, 2022. www.diabetes.org/sites/default/files/2021-10/ADA%20CGM%20Utilization%20White%20Paper.pdf
- Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389-397. doi: 10.2337/dc19-1610
- Vallarino CR, Wong-Jacobson SH, Benneyworth BD, Meadows ES. Costs and outcomes comparison of diabetes technology usage among people with type 1 or 2 diabetes using rapid-acting insulin. J Diabetes Sci Technol. 2021;19322968211052081. doi: 10.1177/19322968211052081
- Wan W, Skandari MR, Minc A, et al. Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care. 2018;41(6):1227-1234. doi: 10.2337/dc17-1821
- Medicare chronic conditions charts. U.S. Centers for Medicare & Medicaid Services. Published August 2021. Accessed May 24, 2022. www2.ccwdata.org/web/guest/medicare-charts/medicare-chronic-condition-charts/#b2_diabetes_current_year
- New Medicare coverage requirements make CGMs more accessible. American Diabetes Association. Accessed May 24, 2022. www.diabetes.org/tools-support/devices-technology/cgm-medicare-coverage-requirement-change-accessibility
- Glucose monitors. U.S. Centers for Medicare & Medicaid Services. Accessed May 24, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
- Modzelewski KL, Murati J, Charoenngam N, Rehm C, Steenkamp DW. Delays in continuous glucose monitoring device initiation: a single center experience and a call to change. Diabetes Technol Ther. 2022. doi: 10.1089/dia.2021.0557
- Engler R, Routh TL, Lucisano JY. Adoption barriers for continuous glucose monitoring and their potential reduction with a fully implanted system: results from patient preference surveys. Clin Diabetes. 2018;36(1):50-58. doi: 10.2337/cd17-0053
- Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181-187. doi: 10.2337/dc16-1536
- Kruger DF, Anderson JE. Continuous glucose monitoring is a tool, not a reward: unjustified insurance coverage criteria limit access to CGM. Diabetes Technol Ther. 2021;23(S3):S45-S55. doi: 10.1089/dia.2021.0193
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi: 10.2337/dci18-0007
- Value-based programs. U.S. Centers for Medicare & Medicaid Services. Updated March 31, 2022. Accessed May 24, 2022. www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs
- AMCP partnership forum: advancing value-based contracting. J Manag Care Spec Pharm. 2017;23(11):1096-1102. doi: 10.18553/jmcp.2017.17342
- Aitken M, Villa P, Tewary V, Anderson A. Innovation in diabetes care technology: value-based agreements. IQVIA Institute for Human Data Science. December 21, 2020. Accessed May 24, 2022. www.iqvia.com/insights/the-iqvia-institute/reports/innovation-in-diabetes-care-technology-value-based-agreements
- Population health management. Academy of Managed Care Pharmacy. Published July 18, 2019. Accessed May 24, 2022. www.amcp.org/about/managed-care-pharmacy-101/concepts-managed-care-pharmacy/population-health-management
- Ferstad JO, Vallon JJ, Jun D, et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: precision health meets population health. Pediatr Diabetes. 2021;22(7):982-991. doi: 10.1111/pedi.13256
- Neilson LM, Swart ECS, Good CB, et al. Identifying outcome measures for type 2 diabetes value-based contracting using the Delphi Method. J Manag Care Spec Pharm. 2019;25(3):324-331. doi: 10.18553/jmcp.2019.25.3.324
- El Malahi A, Van Elsen M, Charleer S, et al. Chronic complications versus glycaemic variability, time in range and HbA1c in people with type 1 diabetes: sub study of the RESCUE-trial. Endocrine Abstracts. 2020;71(012). doi: 10.1530/endoabs.71.012
- Berganstal RM, Hachman-Nielsen E, Kvist K, Buse JB. Derived time-in-range is associated with MACE in T2D: data from the DEVOTE trial. Diabetes. 2020;69(suppl 1). doi: 10.2337/db20-21-LB
- Consensus statement on U.S. health care reform for people with diabetes. Diabetes Leadership Council, Association of Diabetes Care & Education Specialists. Published November 2020. Accessed May 24, 2022. www.diabeteseducator.org/docs/default-source/advocacy/diabetes_health_care_reform_consensus_statement.pdf?sfvrsn=2
- National Committee for Quality Assurance. Rethinking Diabetes Care in the Digital Age. Published February 2, 2022. Accessed May 24, 2022. www.ncqa.org/white-papers/rethinking-diabetes-care-in-the-digital-age/
- Harrington R, Washington D, Paliani S, Thompson K, Rouse L, Anderson AC. A new effort to address racial and ethnic disparities in care through quality measurement. Health Affairs. Published September 9, 2021. Accessed May 24, 2022. www.healthaffairs.org/do/10.1377/forefront.20210907.568444/full/
