- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
Addressing Excessive Evaporation: An Unmet Need in Dry Eye Disease
ABSTRACT
Dry eye disease (DED) is a common condition in which tear film abnormalities result in a damaging cycle of tear hyperosmolarity, desiccating stress, inflammation, and ocular surface injury. In a healthy tear film, meibum produced by the meibomian glands forms a lipid layer that stabilizes the tear film and protects against aqueous tear evaporation. Excessive tear evaporation due to a deficient lipid layer is believed to be the most common cause of DED, and most evaporative DED is associated with meibomian gland dysfunction (MGD); this highlights the pathophysiologic importance of the dysfunctional tear lipid layer. Current treatments for DED may be used to supplement hyperosmolar aqueous tears, lubricate the ocular surface, increase meibum flow, decrease inflammation, promote tear production, or otherwise decrease clinical signs of ocular surface damage and/or improve symptoms. Until now, no prescription eye drop has directly addressed the excessive evaporation that occurs in most patients with DED. Perfluorohexyloctane (PFHO) ophthalmic solution (MIEBO™; Bausch + Lomb) is a preservative-free eye drop that has demonstrated the ability to form a long-lasting barrier that inhibits evaporation in preclinical studies. FDA approval of PFHO was based on results from 2 pivotal clinical trials (GOBI [NCT04139798] and MOJAVE [NCT04567329]) in patients with DED and clinical signs of MGD which demonstrated consistent improvements in both signs and symptoms of disease, with a safety profile similar to that of saline eye drops. PFHO is the first and only FDA-approved eye drop that directly targets tear evaporation in patients with DED, thereby promoting ocular surface healing and providing symptomatic relief.
Am J Manag Care. 2023;29:S239-S247. https://doi.org/10.37765/ajmc.2023.89448
For author information and disclosures, see end of text.
Introduction
Dry eye disease (DED) is a condition in which tear film abnormalities lead to a cycle of tear hyperosmolarity and accompanying ocular surface desiccation, inflammation, and injury.1-3 In the United States, DED is one of the most common ocular conditions noted among patients seeking eye care.1,4
DED is driven by an imbalance between aqueous tear production, quality, and/or evaporation.1 Tear hyperosmolarity and desiccation stress in DED may result from excessive tear evaporation (evaporative dry eye [EDE]), insufficient aqueous tear production at a normal evaporation rate (aqueous-deficient dry eye [ADDE]), or some combination of both factors.1,5
Excessive tear evaporation is a contributing factor in most DED,1,5 and commonly involves meibomian gland dysfunction (MGD).1,5-7 Therapeutic management of DED should be guided by disease etiology and severity, including the presence of MGD.1,6
Up to an estimated 86% of patients with DED have MGD, highlighting the dysfunctional tear lipid layer as a primary driver of DED.5,8,9 Although 1.2 million US adults with DED are treated with a prescription medication,10 millions more are not, and until now, there has been no prescription eye drop that directly addresses the evaporative component of DED.11 In May 2023, the FDA granted approval of a single-component, preservative-free eye drop perfluorohexyloctane (PFHO) ophthalmic solution (MIEBO™; Bausch + Lomb), for the treatment of the signs and symptoms of DED.12,13 Approval was based on clinical trial results supporting the efficacy and safety of PFHO treatment given 4 times daily (qid) to patients with DED and clinical signs of MGD.12-14 PFHO may decrease aqueous tear evaporation by forming a monolayer at the air-liquid interface of the tear film.12,14-16
Physiology of the Ocular Tear Film
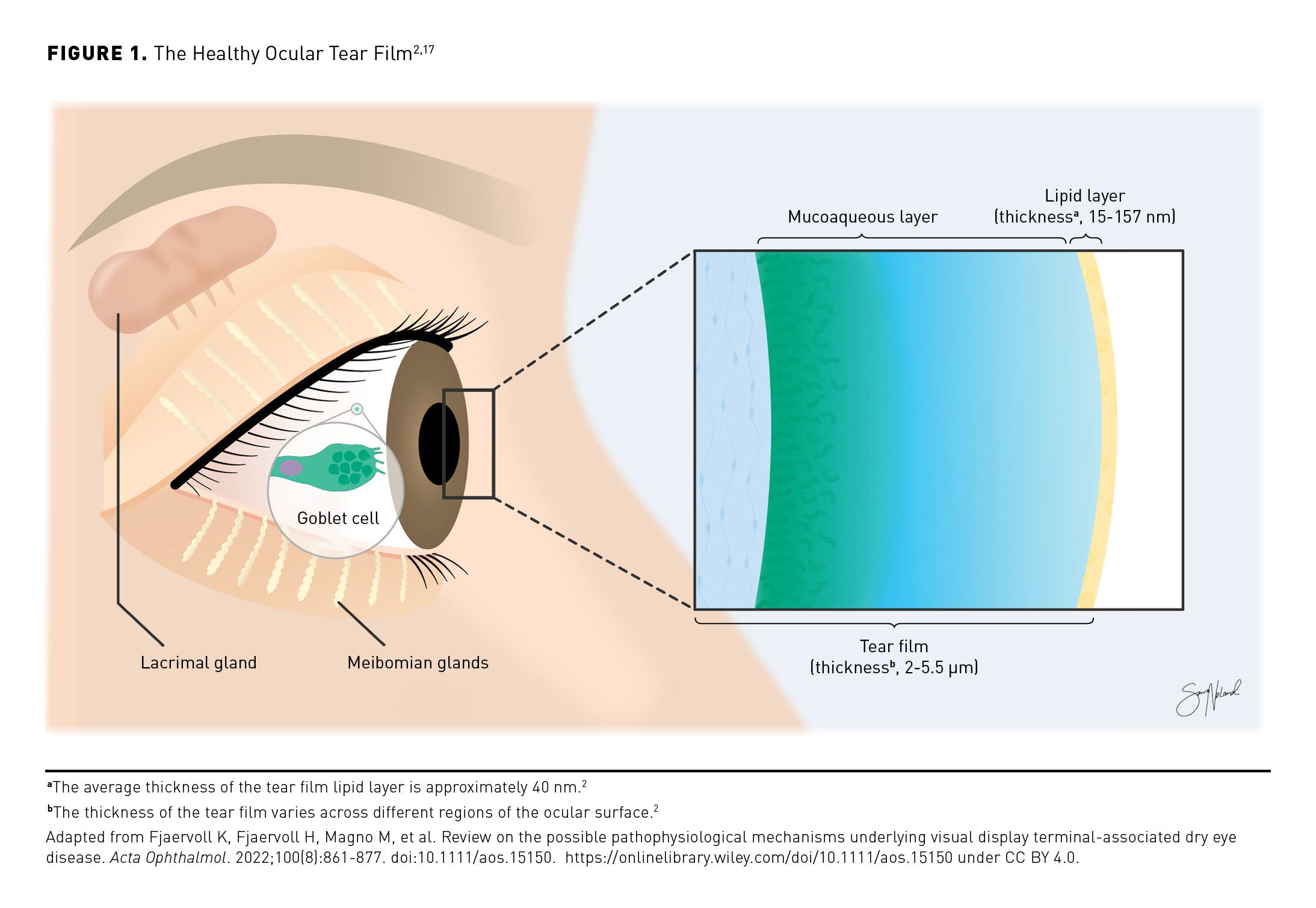
The current model of the ocular tear film indicates that it is composed of 2 layers: a gradient mucoaqueous layer with both bound and free mucus molecules in the aqueous solution that forms the bulk of the tear film and a thin outer lipid layer that resists tear evaporation, protects against foreign matter, and provides a smooth surface for light refraction and reduction of friction while blinking (Figure 1).2,17,18 Aqueous basal tears are produced in the lacrimal glands, and most tear film mucins are synthesized by conjunctival goblet cells.2 The stabilizing outer lipid layer is produced by the meibomian glands, which are located on the upper and lower eyelids in the tarsal plate.2,7 In a healthy tear film, interplay between the lipid layer and an appropriate concentration of proteins in the mucoaqueous layer supports the normal low surface tension required for spreading of the protective tear film across the ocular surface.2
Etiology and Pathophysiology of DED
DED is a multifactorial disease of the ocular surface that may arise consequent to disrupted homeostasis of the ocular tear film.1,11,19 When ocular surface homeostasis is interrupted and evaporation exceeds tear supply, the resulting tear hyperosmolarity and dryness can lead to increased friction, inflammation, and damage of the ocular surface (including loss of corneal/conjunctival epithelial cells and goblet cells, and blockage or loss [dropout] of meibomian glands).1,2,19,20 Other consequences may include stimulation and damage of nerves that contribute to blinking and tear regulation.1,19 These pathophysiologic changes may contribute to further impairment of the tear film that results in ongoing desiccating stress, inflammatory cascades, and further ocular surface injury, which perpetuate the damaging cycle of DED (Figure 2).3,19 This cycle can be self-sustaining once established, regardless of the initial causes.19
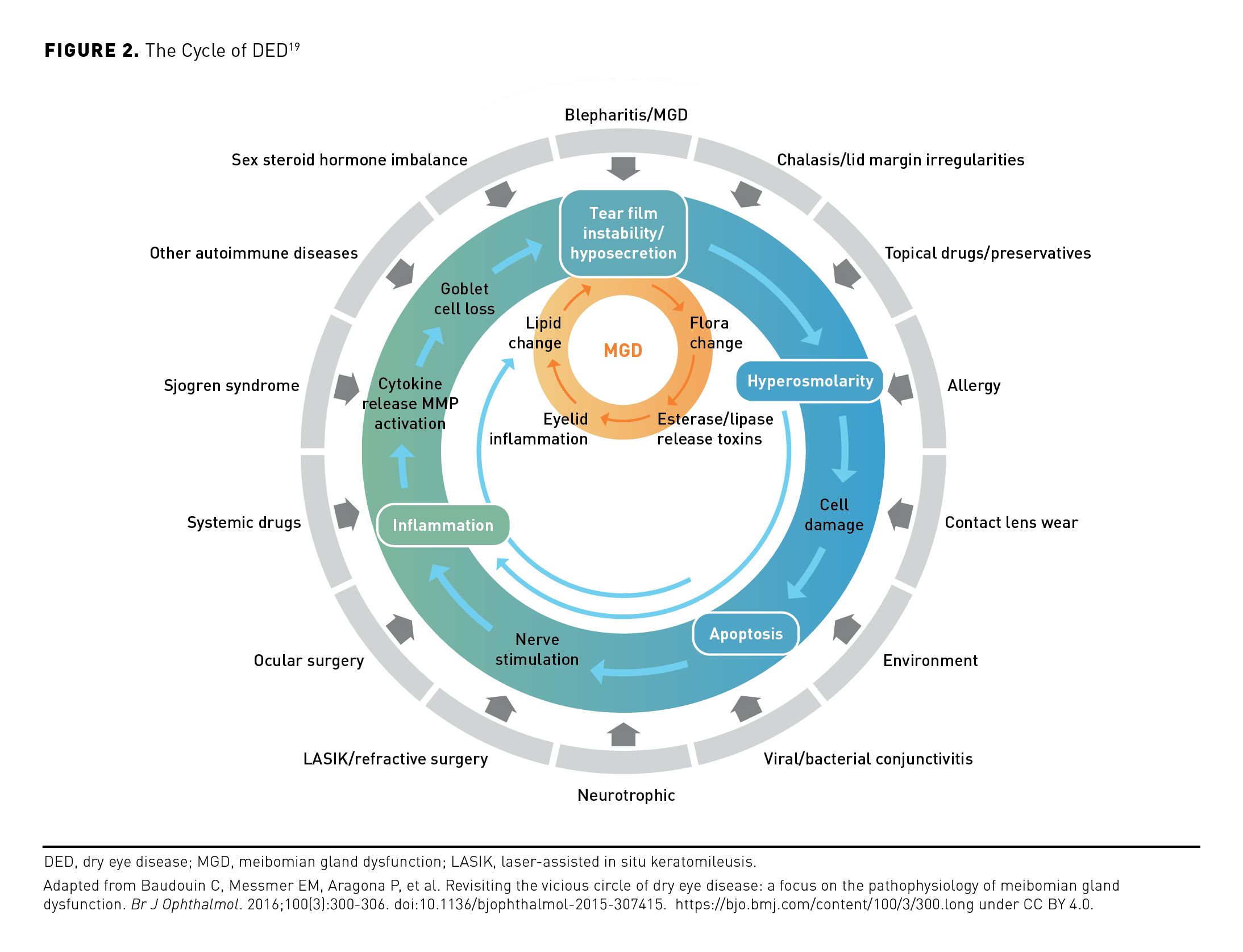
The main driver of DED is dysregulation of tear production accompanied by varying degrees of aqueous tear evaporation.1 This loss of tear film homeostasis and consequent tear hyperosmolarity may develop in eyes with a normal tear evaporation rate if there is insufficient production of the aqueous tear component (ie, ADDE) or if there is excessive tear evaporation (ie, EDE).1,5,21,22 Tear film deficits may be associated with lifestyle factors that may or may not be modifiable, including the use of digital screens, contact lenses, face masks; environment; female sex; older age; continuous positive airway pressure (CPAP) machine use; chronic use of certain medications; and comorbidities such as blepharitis and certain dermatologic conditions, autoimmune diseases, and pain or sleep disorders (Figure 2).1,2,7,8,19,20,23
The Role of MGD in DED
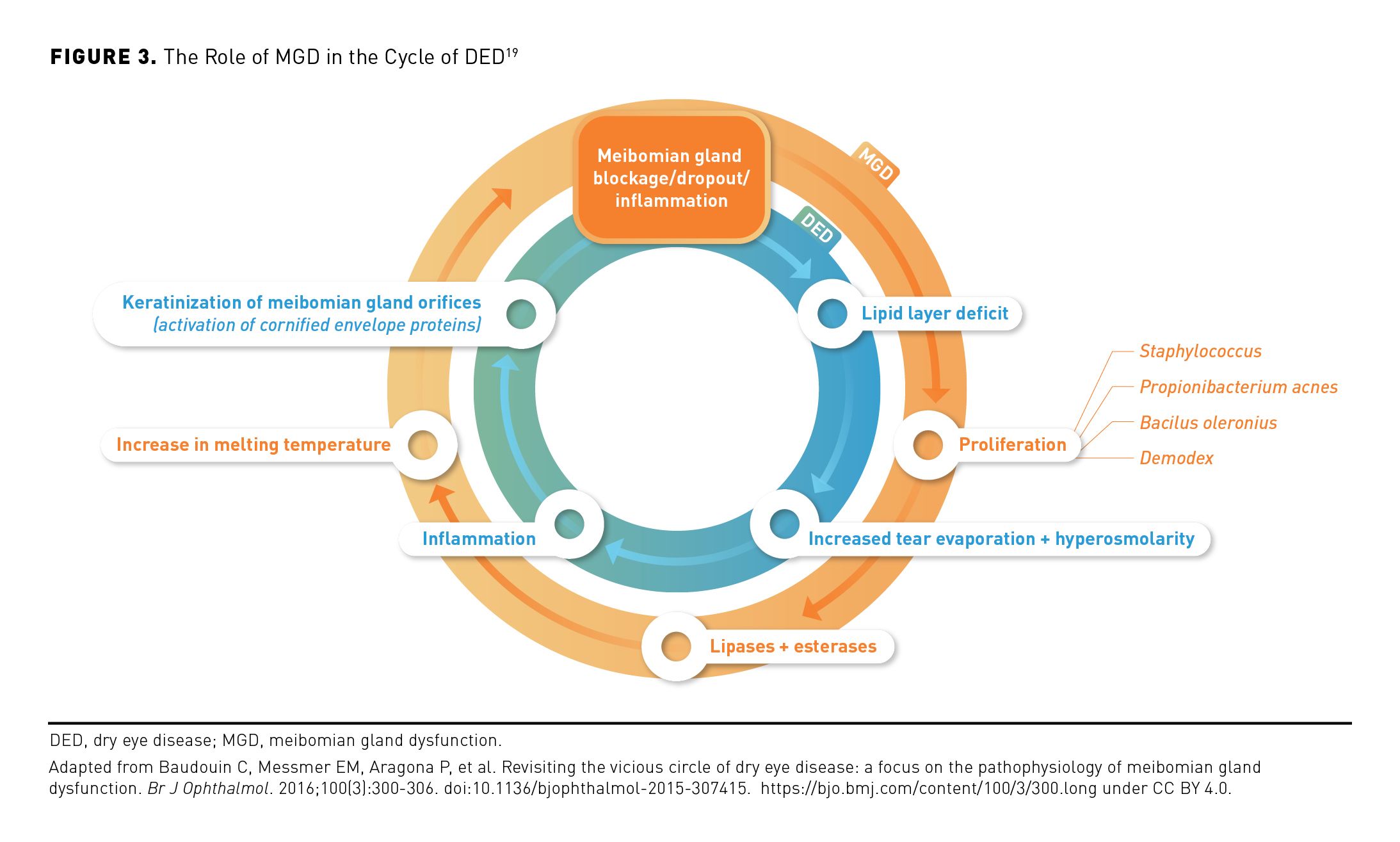
MGD may occur independently of or consequent to DED and often plays a central role in its pathophysiology (Figure 3).19 Inflammation, blockage, or dropout of the meibomian glands promotes meibum insufficiency, triggering the growth of bacteria that can cause changes in meibum lipid composition and increased meibum viscosity, gland inflammation, and hyperkeratinization.19,24,25 These changes may result in reduced meibum secretion onto the ocular surface and continuation of the cycle of MGD.19 The reduced quality or quantity of meibum associated with MGD can result in a deficiency of the tear film lipid layer, increased evaporation, and more rapid tear film disruption, as well as consequent pathophysiologic changes, thus contributing to the damaging cycle of dry eye.1,18,19
Most DED occurs consequent to excessive evaporation rather than being solely associated with a deficit of aqueous tear production; thus, MGD is the main cause of evaporative DED.1,5,26 In an international cohort study, more than 85% of patients with DED were classified as having pure EDE or mixed-etiology DED, whereas less than 15% had pure ADDE.5
Diagnosis of DED and MGD
Formal diagnosis of DED and of MGD may be challenging due to a lack of standardized diagnostic criteria.1,26 According to the Tear Film & Ocular Surface Society’s 2017 Dry Eye Workshop, diagnosis of DED involves triaging questions to rule out conditions that may mimic DED (eg, allergic, bacterial, or viral conjunctivitis; anterior blepharitis; Demodex infestation) and then assessing patient-reported symptoms.1,26,27 Symptomology suggesting DED should trigger an assessment for clinical signs of disease, which may include tear film instability, low tear production, and ocular surface damage.1,7,11,26 Tear film instability may be indicated by a low tear film breakup time [TBUT], low tear production by Schirmer testing, and ocular surface damage via staining (eg, of the cornea, conjunctiva, or lid margin) or assessment of certain biomarkers.1,2,7,22 To guide appropriate treatment decisions, patients may then be assessed for disease severity and DED subtype (ie, EDE [including presence of MGD], ADDE, or mixed).1
Diagnosis of MGD may be complicated by nonspecific presentation of associated symptoms; therefore, evaluation should commence with an assessment of clinical signs of disease.26 Key clinical signs of MGD include compromised lid morphology (assessed via meibography) and low TBUT.1,26 Physical meibomian gland expression to evaluate meibum and assess gland expressibility and dropout may be used to confirm a diagnosis of MGD.7,26
Impact of DED
DED is associated with considerable visual, psychological, social, and economic impact.1,28,29 Patients with DED experience symptoms such as ocular pain or discomfort (eg, dryness, itching, burning), visual fatigue, and blurred vision.1,11,20 DED can be associated with negatively impacted quality of life, including impairment of vision quality, daily activities, and general and mental health.8,26,30 The economic burden of DED is largely driven by indirect costs.29 Increased workplace absenteeism and high rates of productivity loss are often linked to the burden of symptoms and associated quality of life reductions experienced by patients with DED.26,29,31,32
As of 2023, there are estimated to be over 18 million US adults diagnosed with DED; however, approximately 38 million US adults may experience dry eye symptoms.30,33-35 The prevalence of DED increases with patient age, and the number of affected individuals is expected to increase as the American population grows older.4,8,28,34,36,37 Additionally, the annual prevalence of DED is increasing among adults of all ages, with the greatest increase among young adults; this trend may be associated with long-term digital screen use, a lifestyle factor impacting tear evaporation.2,8,20,36,38
Current Treatments
The goal of DED treatment is to reestablish homeostasis of the ocular surface and tear film by addressing the underlying mechanisms of the disease when possible, and long-term treatment is often required to address persistent sequelae.1 Current treatment options may address various pathophysiologic mechanisms (eg, inflammation, aqueous deficiency and hyperosmolarity, lid abnormalities) to disrupt the cycle of disease (Figure 4).3,19
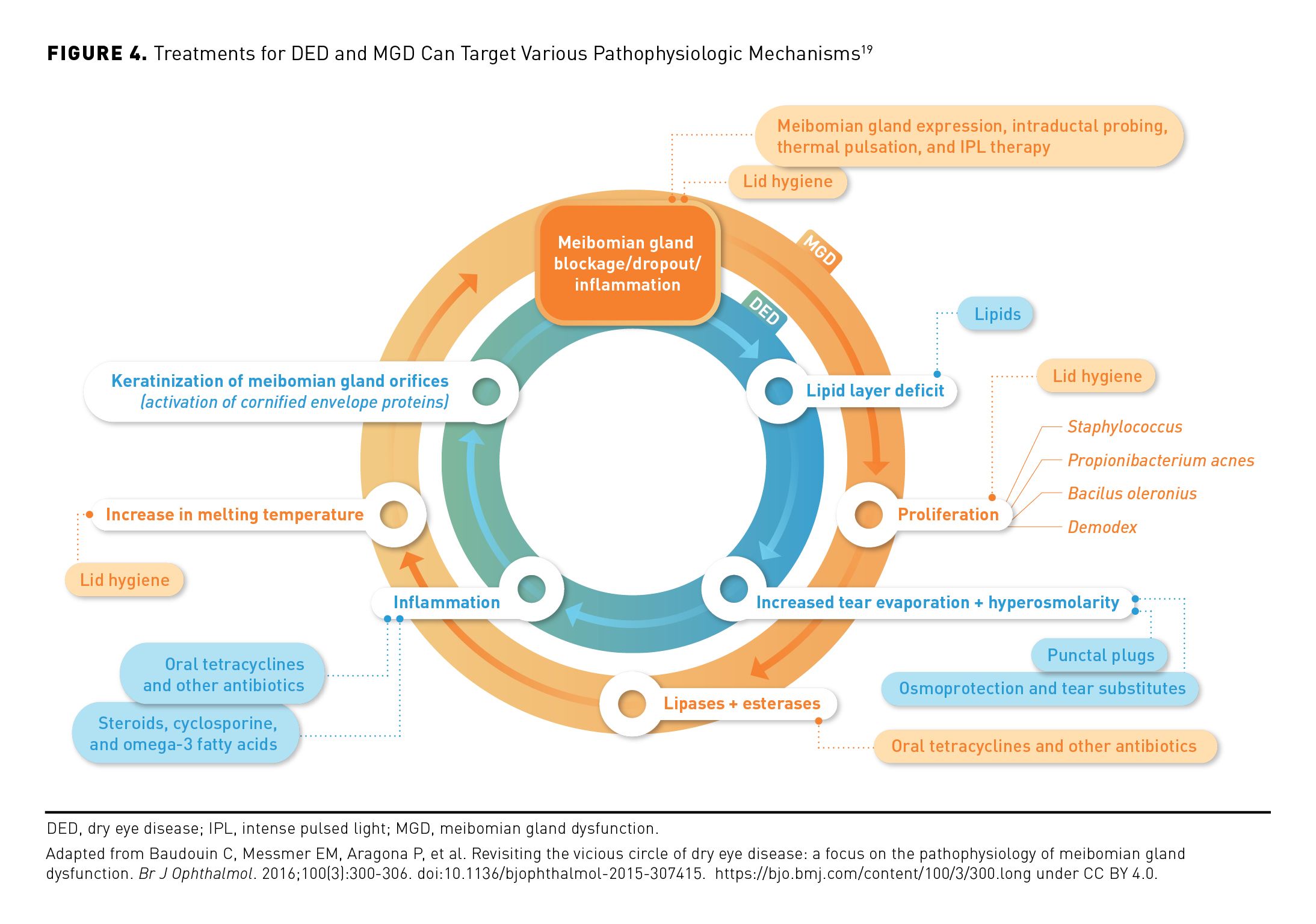
Stepwise treatment is often recommended, starting with low-risk, conservative therapies and proceeding to more advanced treatments with increasing severity or nonresponse to current therapies.1,11 Initial management of mild to moderate DED may include patient education, lifestyle or medication changes (including essential fatty acid supplementation), OTC topical ocular lubricants (eg, artificial tears, including lipid-containing eye drops), and at-home treatments such as eyelid hygiene and warm compresses.1,3
If these approaches prove to be inadequate, office-based procedures or use of prescription medications may be appropriate.1,11 Recommended in-office treatments for DED include placement of temporary or permanent punctal plugs to block nasolacrimal tear drainage thereby increasing tear supply, as well as treatments to improve meibum flow (eg, manual meibomian gland expression, intraductal probing with a microcannula, thermal pulsation or intense pulsed light [IPL] therapies).3,11,38,39 Until recently, prescription medications used for DED treatment included a tear-stimulating nasal spray; a lubricating and tear-film–stabilizing ophthalmic insert; some oral antibiotics with anti-inflammatory effects; and immunomodulatory or anti-inflammatory eye drops containing calcineurin inhibitors, LFA-1 antagonists, corticosteroids, or certain antibiotics to address MGD.1,11,40
The variety of mechanisms of action seen with these drugs may address the wide diversity of pathogenic processes and manifested complications seen in the heterogenous population of patients with DED.1 However, until recently, no FDA-approved prescription eye drop treatments for DED directly targeted excessive evaporation, and approvals were based on results of clinical trials in patients with DED regardless of disease subtype.11,12,41-45
Unmet Needs for The Treatment of DED
The vast majority of DED is associated with excessive evaporation, so the goal of treatment often includes decreasing evaporation to reduce ocular signs and improve patient symptoms.11,26 However, standard prescription eye drops fail to directly address evaporation, and there are a wide variety of known challenges with current home care, OTC, in-office, and prescription treatments. In addition, a lack of high-quality evidence on the relative benefits and long-term efficacy of different treatment options and combination therapies may challenge appropriate and individualized management for patients with DED.3,11,39 Further, there is little research on the comparative efficacy of similar treatment modalities (eg, lid hygiene methods, OTC topical lubricant products, essential fatty acid supplementation protocols) or on the efficacy of various treatments in different DED subtypes.3,39
OTC and Home Care Management
OTC topical lubricating eye drops tend to demonstrate a short-lived ocular surface retention time in patients with DED.11 Among such treatments (low-viscosity artificial tears vs options with viscosity-enhancing components), there is a trade-off between length of symptom relief and effects on vision; eye drops with increased viscosity may reside on the ocular surface for a longer time, but also may be associated with decreased contrast sensitivity.46 In addition, many topical lubricating drops are formulated with preservatives that can cause inflammation and exacerbate DED, particularly with consistent, extended use.11 Furthermore, these OTC treatments are palliative, and they do not address underlying mechanisms of disease.3
Essential fatty acid supplementation is commonly recommended for people with DED, but supportive studies are often of short duration and have shown conflicting results.3,47
Long-term eyelid hygiene is the mainstay of treatment for MGD, but there are no universally accepted guidelines for lid cleansing and eyelid warming procedures.3,11,26,39 Various lid hygiene products (eg, lid scrub, lid wipes, cleansing solutions) are available, and their use often is recommended with twice-daily eyelid warming and massage to promote melting of altered meibum and unblocking of affected glands.11,26,39 Effectiveness of recommended at-home treatments is challenged by patient adherence issues, because their continued therapeutic benefit depends on often time-consuming and labor-intensive maintenance.11,26,39
In-Office Treatments
Meibomian gland expression to improve the tear film lipid layer is an in-office treatment that is often associated with pain or discomfort that limits the force practitioners can use and their ability to remove obstructions.3,39 Intraductal probing is an invasive procedure that can cause lid hemorrhage and may require multiple rounds of treatment.11,39 Punctal plugs may be used to conserve tears and can enhance aqueous tear volume temporarily, but they may not improve tear film stability; further, plastic plugs may be spontaneously extruded, and collagen or silicone plugs dissolve over time.3,38 In addition, thermal pulsation and IPL are in-office treatments that are not covered by insurance; thus, their cost to patients may be substantial.11
Prescription Treatments
Standard topical and systemic treatments prescribed for DED also may be associated with various challenges. Off-label antibiotic therapy (oral and topical) can have anti-inflammatory effects in patients with MGD and thus may improve the dysfunctional tear film lipid layer.11,26 Long-term use of oral antibiotics may be associated with adverse effects (AEs) such as gastrointestinal symptoms.3,11 Treatment with some topical antibiotics may result in improved tolerability and adherence over oral treatments; however, extended use is typically not recommended.3,11
Ophthalmic insert administration to lubricate the eye in patients with moderate to severe DED can lead to corneal abrasion or foreign body sensation if improperly placed; patients may experience temporary vision blurring, and some individuals require twice-daily insertion for sufficient DED symptom relief.40
Use of varenicline nasal spray may be associated with sneezing, coughing, and nose or throat irritation, and requires a somewhat complex multistep process of priming/repriming the bottle and administering the medication.48 This multistep process may be challenging for some patients.
Most available prescription eye drops indicated for treatment of DED either reduce inflammation (corticosteroids and lifitegrast) or increase tear production (cyclosporine) to manage signs and symptoms of disease, but they do not address the root cause of the excessive evaporation that occurs in the majority of patients.1,3,11,41-45 Evidence that patients with DED may show low treatment adherence to cyclosporine- or lifitegrast-containing prescription eye drops and high rates of discontinuing use of these agents suggests a substantial unmet need in the standard treatment landscape.49 Cyclosporine may be associated with the sensation of ocular burning.42 Patients may not experience full clinical benefit for several months, and those already being treated with topical anti-inflammatory drugs or punctal plugs may not experience increased tear production with cyclosporine treatment.3,42,49 In addition, cyclosporine- and lifitegrast-containing eye drops may be administered from single-use vials that can be associated with increased cost.11,42-44
Use of topical corticosteroid eye drops may be associated with ocular hypertension, cataracts, and infections, especially with long-term use.3 For example, the corticosteroid loteprednol etabonate ophthalmic suspension, 0.25%, is only indicated to treat signs and symptoms of DED for up to 2 weeks, although treatment with these eye drops carries a lower risk of intraocular pressure elevation than does treatment with C-20 ketone-based ocular steroids.11,41,50 Loteprednol etabonate and cyclosporine may be used in combination to accelerate clinical improvement.51
Recently approved by the FDA, PFHO is the only prescription eye drop that directly addresses excessive evaporation to reduce the signs and symptoms of DED. PFHO was approved based on consistent results from 2 pivotal clinical trials in patients with a history of DED and clinical signs of MGD.12
Targeting Excessive Evaporation With PFHO
PFHO is a single-component, water-free, preservative-free eye drop that supplements the dysfunctional tear film lipid layer by forming a long-lasting, anti-evaporative layer in patients with DED.15,16,52 PFHO is a semifluorinated alkane with amphiphilic properties that enable it to form a monolayer at the air-liquid interface of the tear film upon instillation.12,15 In preclinical studies, PFHO demonstrated inhibition of the rate of evaporation of saline by approximately 80% (P < .0001), rapid spreading with a low surface tension, and a long residence time at the ocular surface (in rabbits: tears, ≥ 4 hours; meibomian glands, ≥ 8 hours); furthermore, repeated instillation was correlated with progressive improvement in lipid layer thickness and quality (P < .05) in rabbits, and results supported the potential of PFHO to reduce friction at the ocular surface.15,16,52,53
Clinical trial results support the efficacy and safety of topical PFHO treatment in adult patients with DED.13,14,54,55 In the phase 2 SEECASE study, when compared with isotonic saline control, PFHO treatment at 2 different dosing frequencies (bid and qid) was associated with significantly greater changes from baseline in total corneal fluorescein staining (tCFS) scores—a measure of ocular surface damage—and visual analog scale (VAS) mean eye dryness scores (Table).12-14,54,55 Additionally, PFHO treatment was well tolerated, and low rates of instillation site reaction and/or irritation were reported.54
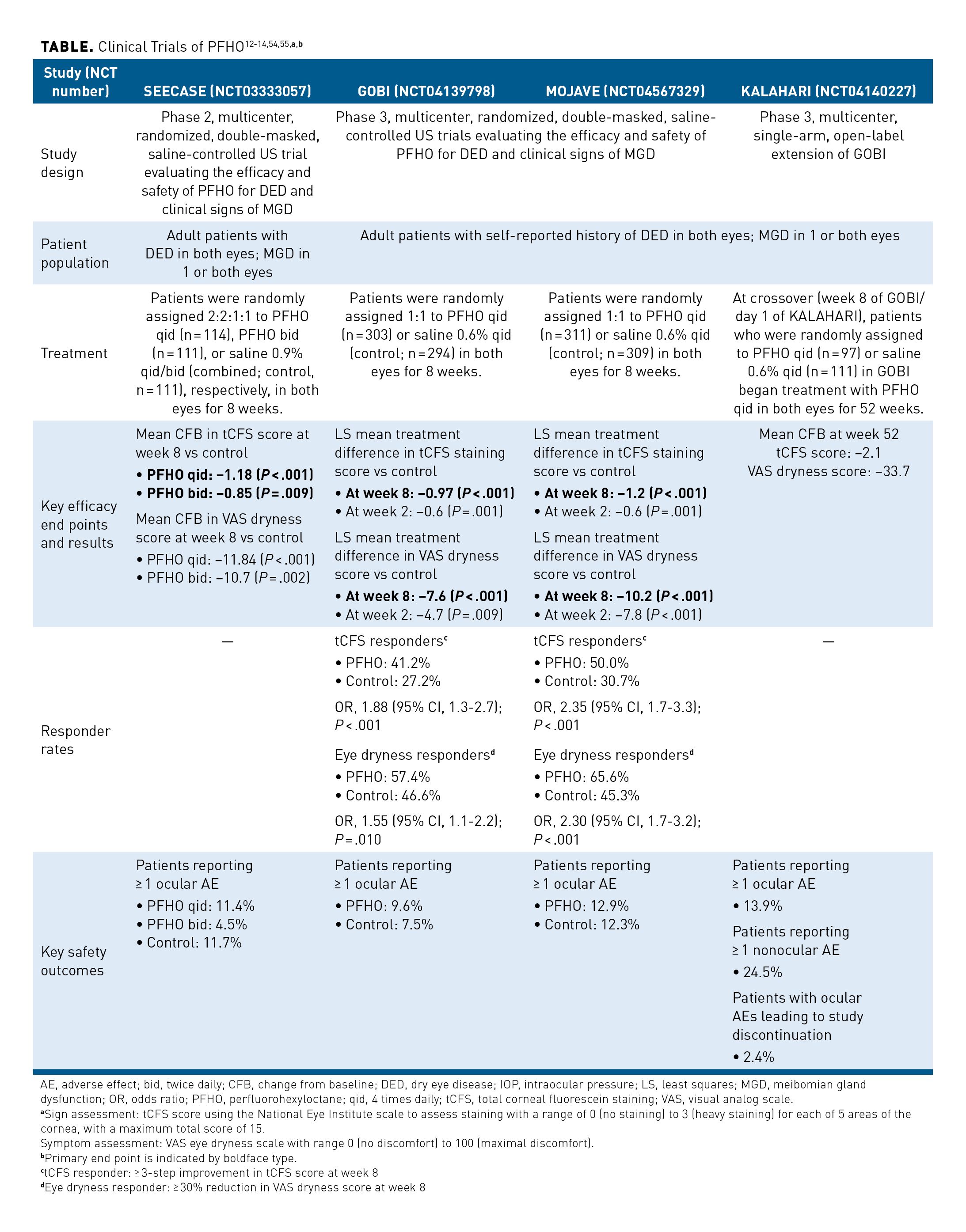
Efficacy and safety of PFHO treatment was further assessed over 8 weeks in the similarly designed phase 3 GOBI and MOJAVE studies.13,14 In both trials, treatment with PFHO qid was associated with significant and clinically meaningful improvements in primary sign and symptom end points at week 8. Compared with use of hypotonic saline, PFHO therapy was associated with significant improvements from baseline in both tCFS and VAS dryness scores at week 2 (the first evaluated time point) and week 8 (the end of the trials) (Table).12-14,54,55 At 8 weeks, the proportions of tCFS responders and eye dryness responders were significantly greater with PFHO treatment than with use of hypotonic saline.13,14 The most commonly reported ocular AEs among patients receiving PFHO included vision blurring (3.0%), instillation-site pain (1.0%), and eye discharge (1.0%) in the GOBI trial, and blepharitis (1.6%), blurred vision (1.3%), conjunctival or ocular hyperemia (both 1.3%), conjunctival papillae (1.3%), visual acuity reduction (1.0%), and hordeolum (1.0%) in the MOJAVE trial.The low rate of ocular AEs was similar between treatment arms in both trials and were of mostly mild severity, confirming the findings from SEECASE that PFHO treatment was very well tolerated.13,14,54
In addition, results from the KALAHARI extension trial support the consistent efficacy and safety of PFHO treatment in patients with DED and clinical signs of MGD. KALAHARI was a 52-week, phase 3 extension of the GOBI trial. Long-term treatment with PFHO was well tolerated, with mostly mild AEs reported. The improvements in tCFS and VAS dryness scores seen in the active arm of the GOBI study were maintained through 52 weeks of PFHO treatment in the KALAHARI trial. Among control patients in the GOBI trial who switched over to PFHO treatment at the start of the KALAHARI study, improvements in these measures were seen by week 4 and were maintained for the rest of the study.55
In the SEECASE, GOBI, and MOJAVE clinical trials, topical PFHO dosed qid consistently showed improvements in measures of both ocular surface damage and eye dryness as compared to the control (saline) in patients with DED and clinical signs of MGD; further, safety findings were similar to that of saline eye drops. Taken together, results from the GOBI trial and KALAHARI extension study indicate that PFHO qid exhibits a favorable safety profile and is efficacious when used for periods of up to 15 months.
Conclusions
The most common cause of DED is MGD, which results in a deficient lipid layer, tear film instability, and excessive evaporation, thereby perpetuating a damaging cycle of hyperosmolarity, inflammation and ocular surface injury. Until now, no prescription eye drop has directly addressed the excessive evaporation that is a core pathophysiologic mechanism underlying DED. PFHO supplements the dysfunctional lipid layer by forming a long-lasting barrier on the tear film surface that reduces evaporation to help break the cycle of DED, allowing ocular surface healing and symptom relief.
The results of multiple clinical studies found that PFHO treatment was consistently associated with improvements in signs and symptoms of DED. These significant, clinically meaningful improvements were consistent over 15 months, and PFHO treatment was very well tolerated. With its recent FDA approval, PFHO may address many of the unmet needs in the treatment landscape of DED.
Authorship affiliation: Kentucky Eye Institute Lexington (PMK); University of Alabama at Birmingham (KKN); Eastern Virginia Medical School (JDS)
Funding source: Financial support for this article was provided by Bausch + Lomb.
Author disclosure: Dr Karpecki reports serving on a consultancy or paid advisory board for AbbVie, Alcon, Allergan, Bausch + Lomb, Harrow Health, Oasis Medical, OcuSoft, Scope, Sight Sciences, Sun Pharmaceuticals, and Viatris. Dr Karpecki also reports receiving lecture fees for speaking at the invitation of a commercial sponsor from Bausch + Lomb, Dompé, Mallinckrodt, Sun Pharmaceuticals, and Viatris. Dr Nichols reports serving on a consultancy or paid advisory board for AbbVie, Aerie, Alcon, Alderya, Allergan, Axim, Azura, Bausch + Lomb, Bruder, Cavalry, Dompé, HanAll Biopharma, Nicox, Novaliq, Novartis, Osmotica, Oyster Point Pharma, Palatin, RVL, Shire, Takeda, Tarsus, TearSolutions, Thea, TopiVert, Trukera, Versea, and Xequel. Dr Nichols also reports receiving grants from Aramis, Kowa, ScienceBased Health, Sylentis, and TearScience, and receiving lecture fees for speaking at the invitation of a commercial sponsor from Bausch + Lomb and Dompé. Dr Sheppard reports serving on a consultancy or paid advisory board for AbbVie, Bausch + Lomb, Dompé, Mallinckrodt, and Viatris. Dr Sheppard also reports receiving lecture fees for speaking at the invitation of a commercial sponsor from AbbVie, Bausch + Lomb, Dompé, Mallinckrodt, and Viatris.
Authorship information: Concept and design (PMK, KKN, JDS); analysis and interpretation of data (PMK, JDS); drafting of the manuscript (PMK,KKN); critical revision of the manuscript for important intellectual content (PMK, KKN, JDS); provision of study materials of patients (JDS); administrative, technical or logistic support (JDS); supervision (JDS).
Address correspondence to: Paul M. Karpecki, OD, FAAO. Kentucky Eye Institute, 601 Perimeter Drive, Suite 100. Lexington, KY 40517. karpecki@karpecki.com
References
- Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-812. doi:10.1016/j.jtos.2017.08.003
- Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366-403. doi:10.1016/j.jtos.2017.03.006
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. doi:10.1016/j.jtos.2017.05.006
- Bradley JL, Özer Stillman I, Pivneva I, Guerin A, Evans AM, Dana R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin Ophthalmol. 2019;13:225-232. doi:10.2147/OPTH.S188314
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478. doi:10.1097/ICO.0b013e318225415a
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283. doi:10.1016/j.jtos.2017.05.008
- Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929. doi:10.1167/iovs.10-6997a
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-365. doi:10.1016/j.jtos.2017.05.003
- Rabensteiner DF, Aminfar H, Boldin I, Schwantzer G, Horwath-Winter J. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96(6):e707-e711. doi:10.1111/aos.13732
- Longitudinal Access and Adjudication Data, August 2020-July 2021. Unique dry eye patients with claims for Restasis, Cequa, Eysuvis, Lacrisert, Tyrvaya or Cyclosporine. IQVIA.
- Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-1418. doi:10.1007/s40123-023-00669-1
- Miebo. Prescribing information. Bausch + Lomb; 2023. Accessed August 17, 2023. https://www.bausch.com/globalassets/pdf/packageinserts/pharma/miebo-package-insert.pdf
- Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524. doi:10.1016/j.ophtha.2022.12.021
- Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265-274 doi:10.1016/j.ajo.2023.03.008
- Vittitow J, Kissing R, DeCory H, Borchman D. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res. 2023;29:100704. doi:10.1016/j.curtheres.2023.100704
- Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17(2):241-249. doi:10.1016/j.jtos.2019.02.010
- Fjaervoll K, Fjaervoll H, Magno M, et al. Review on the possible pathophysiological mechanisms underlying visual display terminal-associated dry eye disease. Acta Ophthalmol. 2022;100(8):861-877. doi:10.1111/aos.15150
- Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20-S26. doi:10.1016/j.ophtha.2017.05.031
- Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300-306. doi:10.1136/bjophthalmol-2015-307415
- Nebbioso M, Del Regno P, Gharbiya M, Sacchetti M, Plateroti R, Lambiase A. Analysis of the pathogenic factors and management of dry eye in ocular surface disorders. Int J Mol Sci. 2017;18(8):1764. doi:10.3390/ijms18081764
- McMonnies CW. Aqueous deficiency is a contributor to evaporation-related dry eye disease. Eye Vis (Lond). 2020;7:6. doi:10.1186/s40662-019-0172-z
- Hakim FE, Farooq AV. Dry Eye Disease: An Update in 2022. JAMA. 2022;327(5):478-479. doi:10.1001/jama.2021.19963
- Galor A, Britten-Jones AC, Feng Y, et al. TFOS lifestyle: impact of lifestyle challenges on the ocular surface. Ocul Surf. 2023;28:262-303. doi:10.1016/j.jtos.2023.04.008
- Khanal S, Ngo W, Nichols KK, Wilson L, Barnes S, Nichols JJ. Human meibum and tear film derived (O-acyl)-omega-hydroxy fatty acids in meibomian gland dysfunction. Ocul Surf. 2021;21:118-128. doi:10.1016/j.jtos.2021.05.009
- Nagar S, Ajouz L, Nichols KK, et al. Relationship between human meibum lipid composition and the severity of meibomian gland dysfunction: a spectroscopic analysis. Invest Ophthalmol Vis Sci. 2023;64(10):22. doi:10.1167/iovs.64.10.22
- Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179-192. doi:10.1016/j.jtos.2017.01.006
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-574. doi:10.1016/j.jtos.2017.05.001
- Clayton JA. Dry eye. N Engl J Med. 2018;378(23):2212-2223. doi:10.1056/NEJMra1407936
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379-387. doi:10.1097/ICO.0b013e3181f7f363
- Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806. doi:10.1016/j.ajo.2013.12.023
- Nichols KK, Bacharach J, Holland E, et al. Impact of dry eye disease on work productivity,
and patients’ satisfaction with over-the-counter dry eye treatments. Invest Ophthalmol Vis Sci. 2016;57(7):2975-2982. doi:10.1167/iovs.16-19419 - Morthen MK, Magno MS, Utheim TP, Hammond CJ, Vehof J. The work-related burden of dry eye.
Ocul Surf. 2023;28:30-36. doi:10.1016/j.jtos.2023.01.006 - Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98. doi:10.1016/j.ajo.2017.06.033
- U.S. and world population clock. United States Census Bureau. Accessed August 17, 2023.
https://www.census.gov/popclock/ - National population by characteristics: 2-20-2022. Population estimates by age (18+): July 1, 2022 (SCPRC-EST2022-18+POP). United States Census Bureau. Revised March 31, 2023. Accessed August 17, 2023. https://www2.census.gov/programs-surveys/popest/tables/2020-2022/state/detail/SCPRC-EST2022-18+POP.xlsx
- Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States health care system. Am J Ophthalmol. 2019;202:47-54. doi:10.1016/j.ajo.2019.01.026
- Wang MTM, Muntz A, Lim J, et al. Ageing and the natural history of dry eye disease: a prospective registry-based cross-sectional study. Ocul Surf. 2020;18(4):736-741. doi:10.1016/j.jtos.2020.07.003
- Donthineni PR, Shanbhag SS, Basu S. An evidence-based strategic approach to prevention and treatment of dry eye disease, a modern global epidemic. Healthcare (Basel). 2021;9(1):89. doi:10.3390/healthcare9010089
- Arita R, Fukuoka S. Non-pharmaceutical treatment options for meibomian gland dysfunction. Clin Exp Optom. 2020;103(6):742-755. doi:10.1111/cxo.13035
- Lacrisert. Prescribing information. Bausch + Lomb; 2019. Accessed August 17, 2023.
https://www.lacrisert.com/siteassets/pdf/Lacrisert-package-insert.pdf - Eysuvis. Prescribing information. Kala Pharmaceuticals; 2020. Accessed August 17, 2023.
https://www.eysuvis-ecp.com/pdf/prescribing-information.pdf - Restasis. Prescribing information. Allergan; 2017. Accessed August 17, 2023. https://www.rxabbvie.com/pdf/restasis_pi.pdf
- Xiidra. Prescribing information. Novartis Pharmaceuticals; 2020. Accessed August 17, 2023.
https://www.novartis.com/us-en/sites/novartis_us/files/xiidra.pdf - Cequa. Prescribing information. Sun Pharmaceutical; 2022. Accessed August 17, 2023.
https://www.cequa.com/CequaPI.pdf - Vevye. Prescribing information. Novaliq; 2023. Accessed August 24, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217469s000lbl.pdf
- Hall JQ Jr, Ridder WH 3rd, Nguyen AL, Paugh JR. Visual effect and residence time of artificial tears in dry eye subjects. Optom Vis Sci. 2011;88(7):872-880. doi:10.1097/OPX.0b013e31821b0b2c
- Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. 2019;12(12):CD011016. doi:10.1002/14651858.CD011016.pub2
- Tyrvaya. Prescribing information. Viatris; 2021. Accessed August 17, 2023.
https://www.tyrvaya-pro.com/files/prescribing-information.pdf - White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;13:2285-2292. doi:10.2147/OPTH.S226168
- Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther. 2016;33(4):532-552. doi:10.1007/s12325-016-0315-8
- Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation
of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289-296.
doi:10.1097/ICL.0000000000000049 - Krösser S, Spencer E, Grillenberger R, et al. Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits. Poster presented at: Association for Research in Vision and Ophthalmology 2018 Annual Meeting; April 29 to May 3, 2018; Honolulu, HI. Accessed August 17, 2023. https://www.novaliq.com/wp-content/uploads/2021/01/07_Kroesser_ARVO2018_radiolabel-distribution-rabbits_EyeSol.pdf
- Agarwal P, Khun D, Krösser S, et al. Evaluating the lubricating effect of semifluorinated alkanes on the ocular surface. Invest Ophthalmol Vis Sci. 2018;59(9):3282.
- Tauber J, Wirta DL, Sall K, et al; SEECASE study group. A randomized clinical study (SEECASE) to assess efficacy, safety, and tolerability of NOV03 for treatment of dry eye disease. Cornea. 2021;40(9):1132-1140. doi:10.1097/ICO.0000000000002622
- Protzko E, Segal B, Korenfeld M, Krösser S, Vittitow J. Long-term safety and efficacy of perfluorohexyloctane ophthalmic solution for the treatment of patients with dry eye disease: the KALAHARI study. Cornea. In press.
