- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
A Primer on Cervical Cancer and Current Standards of Care
Cervical cancer is the fourth most common cancer in women in the United States. As of 2011, the estimated annual medical cost of care is approximately $1.6 billion.1-4 For individual patients during their last year of life, these costs can be as high as $79,000 for those older than 65 years and $118,000 for younger patients.3,4
Most cases of cervical cancer can be prevented by human papillomavirus (HPV) vaccination, routine screening, and treatment of precancerous lesions.1,5,6 In fact, incidence rates are higher in areas where there is less access to screening. During the COVID-19 pandemic, there was a considerable decline in screening rates for cervical cancer. This decline was seen everywhere in the country and ranged from 77% in urban areas to 82% in metropolitan areas and 85% in rural areas. The most affected ethnic groups were Asian/Pacific Islander women (92%) and Black women (82%).7
Survival rates vary depending on how advanced the disease is; localized and early-stage disease have high survival rates, whereas patients with advanced and recurring disease have much poorer outcomes with the available therapies, highlighting the necessity for the expansion of treatment options.8 This article provides an overview of the incidence, pathogenesis, and treatment of cervical cancer, including the current standard of care (SOC) for patients with advanced or recurring disease.
Incidence and Risk Factors
In the United States, there has been a 50% decrease in incidence and mortality rates of cervical cancer that began in the 1970s when cervical cancer screening became widespread.3,5,6,9,10 The trend of decreased rates continued following the availability and uptake of the first HPV vaccine in 2006.3,5,6,9-11
However, the pace of this decline has slowed during the past decade.3,9,10 In 2018, the CDC reported 12,733 new cases of cervical cancer and about 4000 deaths. It is estimated that in 2021, these numbers will increase to 14,480 new cases and 4290 deaths.3,9,12,13
Although infections with HPV are common and rarely cause cancer, nearly all cervical cancers are caused by persistent infection with certain types of the virus.9-15 In addition to persistent infection with HPV, other risk factors include tobacco smoking, long-term use of oral contraceptives, immunosuppression, HIV infection, young age at first sexual encounter, high number of sexual partners, exposure to diethylstilbestrol in utero, chlamydia infection, and multiple full-term pregnancies beginning at a young age.10,14,16-25
Pathogenesis, Staging, and Prognosis
There are 2 main types of cervical cancers: squamous cell carcinoma (SCC) and adenocarcinoma (ADC).16,26 About 80% to 90% of cervical cancers are SCCs that develop from cells in the exocervix, and 10% to 20% are ADCs that develop from the mucus-producing gland cells of the endocervix. Some tumors can also present features of both types and are called adenosquamous or mixed carcinomas.
Most cervical cancers develop in the transformation zone, which is the junction between the ectocervix and the endocervical canal.16 Precancerous lesions present as intraepithelial neoplasia and are named either cervical intraepithelial neoplasia or ADC in situ. If left untreated, these lesions eventually progress to invasive cancer.10,16 This progression is typically slow (10-12 years) for most patients (30%-70%). However, for about 10% of patients, some lesions can progress in less than a year.27
As mentioned above, HPV infection is the main causal factor for cervical cancer, and 90% of cervical cancers are positive for HPV DNA.10,28 HPV mainly targets squamous epithelium and is linked to cutaneous and mucosal infections.17 There are 14 high-risk HPV types that are associated with a higher risk of cervical cancer development, and most HPV-related cancers are caused by HPV-16 and HPV-18.29,30
The immune system is often able to fight off HPV infections before they cause serious cell damage and harm, but sometimes, HPV can evade detection by the immune system. Persistent HPV infections may cause normal cells on the surface of the cervix to transform into malignant cells, which could result in cervical cancer.17,31
Cervical cancer may not initially present with any signs or symptoms.1,17,32 Screening for cervical cancer involves a complete medical history, a physical and pelvic exam, and cervical cytology (Papanicolaou test). Abnormal findings can be further investigated with colposcopy, endocervical curettage, or biopsy.17,32
Based on the results from the diagnostic tests, which include the physical exam, biopsies, and imaging tests, the clinical stage of the disease can be determined.33 Cervical cancer is classified according to the International Federation of Gynecology and Obstetrics (FIGO) staging system as either stage I (confined to the cervix), stage II (extended beyond the uterus but not up to the pelvic wall), stage III (extended up to the pelvic wall and/or involving the pelvic and/or para-aortic lymph nodes), or stage IV (extended beyond the pelvis).34,35
The prognosis depends on several factors, which include the clinical stage of the disease at the time of diagnosis and pelvic lymph node status.36 Other factors that are associated with poor outcomes include positive HIV status, c-MYC overexpression, and HPV-18 infection.36-40
According to data from the Surveillance, Epidemiology, and End Results database, the average 5-year relative survival rate among all patients with cervical cancer (based on diagnoses between 2011 and 2017) is 66%. The relative survival rate is 92% for patients with localized disease (cancer has not spread outside the cervix or uterus), 58% for patients with regional disease (cancer has spread to nearby lymph nodes), and 18% for patients with distal disease (cancer has spread outside the cervix and uterus to nearby or distant organs).41
Considerations for Treatment Decision-Making
The current SOC treatments for cervical cancer are summarized in the Table.27 The most important factor when choosing a treatment for cervical cancer is the FIGO stage. Other factors that may also influence this decision include the type of cancer (SCC or ADC), the exact location of the tumor, the age of the patient and their overall health, and the patient’s wish to preserve their fertility.1,42
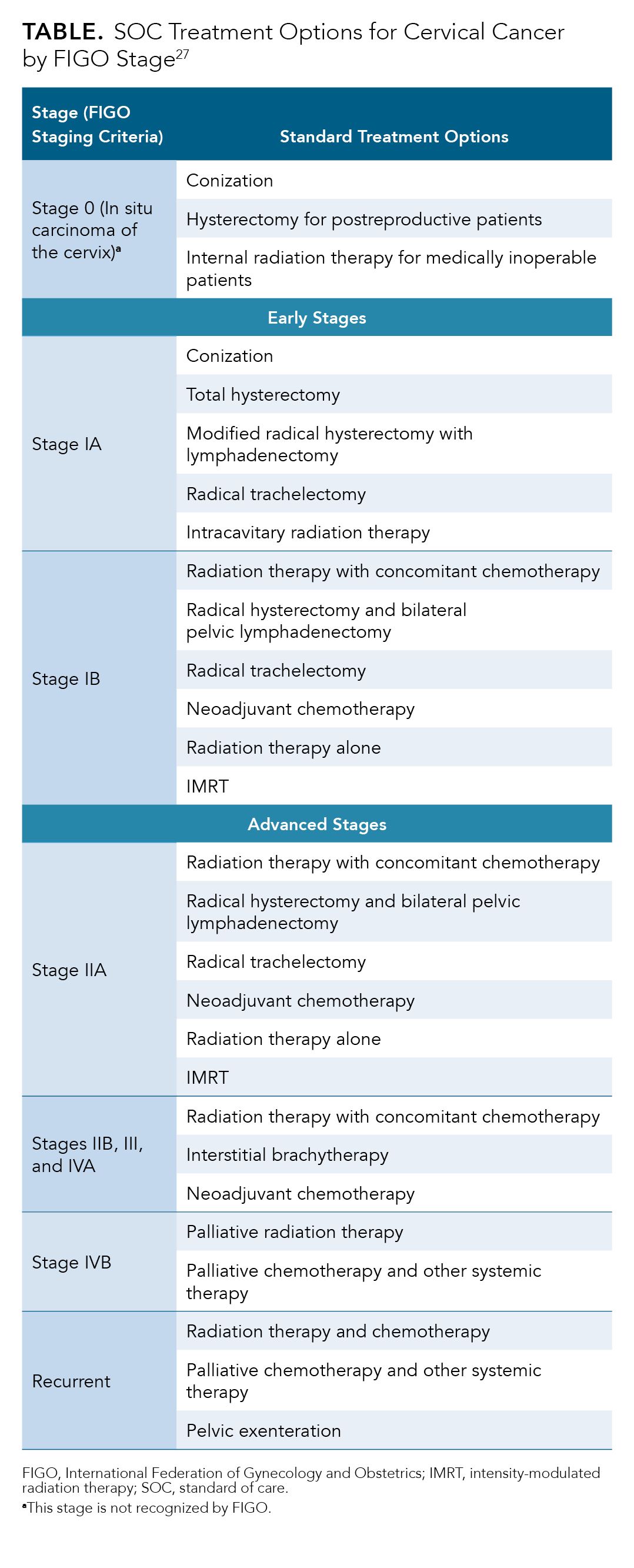
Typically, early-stage disease (stages IA, IB1, and IB2) can be treated with surgery.42 Fertility preservation options (conization and trachelectomy) are available for patients with up to stage IB2 cervical cancer.27,42 There is disagreement about whether adjuvant hysterectomy following primary chemoradiation should be recommended for stage IB or IIA tumors, as there is limited evidence of benefits and some evidence of increased morbidity associated with the procedure.27,42-45 If pathologic risk factors, such as large primary tumors, deep stromal invasion, lymphovascular space invasion, positive lymph nodes, surgical margins, or parametrium, are discovered following radical hysterectomy, adjuvant treatment with radiotherapy alone or chemoradiotherapy is recommended.27,42
Radiation therapy is another appropriate option for early-stage disease.46 Typically, external beam radiation therapy (EBRT) is administered to the pelvis (45-50 Gy) together with low-dose brachytherapy, if necessary. The traditional radiation therapy dose is 70 Gy for smaller tumors and up to 90 Gy for larger ones.27
Concurrent chemoradiation, a combination of platinum-containing chemotherapy and radiation therapy, is recommended for advanced-stage cervical cancer (stages IB3 through IVA), but can also be used for patients with earlier stages of the disease who are not candidates for hysterectomy.42 Chemoradiation is superior to radiation alone in terms of overall and disease-free survival.47,48 Cisplatin as a single agent is the preferred chemotherapy regimen and can be substituted with carboplatin if the patient is cisplatin intolerant.27,42,49-51 Cisplatin/fluorouracil is an alternative first-line regimen, although it may have higher toxicity.51,52 There is evidence that cisplatin/gemcitabine used concurrently with EBRT, followed by 2 additional cycles of chemotherapy after radiation therapy, may improve survival compared with survival after cisplatin therapy alone.53 However, this regimen also has increased toxicity. Finally, there are nonplatinum-based options such as fluorouracil and/or mitomycin for patients who are unable to tolerate platinum-based therapy.54
Neoadjuvant chemotherapy followed by surgery can be used for stages IB through IVA. There have been several studies investigating the role of neoadjuvant chemotherapy, and the largest randomized trial showed an improvement in survival for patients with stages IB, IIA, and IIB cervical cancer. However, the results are not reflective of current practice, and the control arm used radiation therapy alone.27
Finally, in the past few years, targeted agents and biologics have been developed for the treatment of various cancers, including cervical cancer. Two of these agents, bevacizumab and pembrolizumab, are indicated for the treatment of cervical cancer.55,56
Advanced and Recurrent Cervical Cancer
Response rates of various SOC regimens for recurrent cervical cancer range from 11% with bevacizumab monotherapy to 46% with cisplatin/paclitaxel combination.27 Because the survival rate for patients with distal disease is 18%, there is a need for therapies that can adequately treat advanced and recurrent cervical cancer.41 Treatment for recurrent disease depends on whether the recurrence is localized or distant.42
In general, patients with localized recurrences may be candidates for radical retreatment with radiation therapy and/or chemotherapy or with surgery, depending on the nature of the original treatment and where the recurrent tumor presents. If surgery is feasible, it should be considered; radiation therapy with or without chemotherapy is also an option.27,42 The preferred chemotherapy regimens are single-agent cisplatin, carboplatin, paclitaxel, or topotecan. Other agents include docetaxel, ifosfamide, 5-fluorouracil, irinotecan, gemcitabine, and mitomycin, and drug combinations are often used.42
The prognosis for patients who experience recurrence and for those who develop distant metastases is poor.27,42 The recommended treatment for patients with metastases typically includes platinum-based chemotherapy (cisplatin or carboplatin) combined with other agents such as paclitaxel and topotecan. Bevacizumab in combination with paclitaxel and cisplatin or paclitaxel and topotecan is also indicated for patients with persistent, recurrent, or metastatic cervical cancer, and its addition provides a survival benefit.27,55 While cisplatin-based chemoradiotherapy treatments for early-stage disease are effective, the response rates in patients with advanced or recurrent disease range from 18% for single-agent use to 46% when combined with paclitaxel.57,58 Finally, there are clinical trials available for patients with metastatic or recurrent disease.27,42
Bevacizumab is a monoclonal antibody that targets the vascular endothelial growth factor, which promotes angiogenesis and is a key mediator of cancer progression.59,60 In 2014, the Gynecologic Oncology Group (GOG) 240 phase 3 clinical trial (NCT00803062) assessed the efficacy and safety of adding bevacizumab to combination chemotherapy for advanced or recurrent cervical cancer. The study enrolled 452 patients with metastatic, persistent, or recurrent cervical carcinoma, who were randomized to receive cisplatin plus paclitaxel with or without bevacizumab or topotecan plus paclitaxel with or without bevacizumab. All patients had to have a GOG performance status of 0 or 1; adequate renal, hepatic, and bone marrow function; and measurable disease. Ineligibility criteria included patients who were candidates for curative therapy by pelvic exenteration, those receiving chemotherapy for recurrence, and those with nonhealing wounds, active bleeding conditions, or inadequately anticoagulated thromboembolism. The primary end points for this study were overall survival (OS) and frequency and severity of adverse events (AEs); the secondary end points were progression-free survival (PFS) and response rate.61
At the 20.8-month follow-up, the median OS was 3.7 months longer (17.0 vs 13.3 months) for the groups that incorporated bevacizumab (HR for death, 0.71; 98% CI, 0.54-0.95). There was also improvement in PFS (8.2 vs 5.9 months), and the response rate was higher (48% vs 36%) in the bevacizumab groups.61
The main AEs of concern that were higher in the bevacizumab groups were grade 3 or higher gastrointestinal or genitourinary fistulas (6% vs 0%), thromboembolic events (8% vs 1%), and grade 2 hypertension (25% vs 2%).61 Based on these results, bevacizumab combined with paclitaxel and cisplatin or paclitaxel and topotecan received FDA approval to treat patients with persistent, recurrent, or metastatic cervical cancer.55
In 2018, pembrolizumab, a monoclonal antibody against PD-1, received approval by the FDA for the treatment of recurrent or metastatic cervical cancer on or after chemotherapy whose tumors express PD-L1 (combined positive score [CPS] ≥ 1).62,63 PD-1 is a receptor expressed on the surface of T cells that acts in an immunoregulatory capacity and can contribute to carcinogenesis by allowing tumors to evade immunosurveillance.63-65
One of the pivotal trials leading to the approval of pembrolizumab as a treatment for cervical cancer was KEYNOTE-158 (NCT02628067), a phase 2 basket study that investigated the efficacy and safety of pembrolizumab in adult patients with previously treated advanced solid tumors.62,65 Patients in the cervical cancer cohort (N = 38) had measurable disease confirmed histologically or cytologically, progression during or intolerance to standard therapies, an ECOG performance status of 0 or 1, and adequate organ function. PD-L1 expression was assessed from a new or archived nonirradiated tumor sample; PD-L1 positivity was defined as CPS ≥ 1. Exclusion criteria included active central nervous system metastases; active autoimmune disease; history of noninfectious pneumonitis; prior targeted therapy against PD-1, PD-L1, PD-L2, or another coinhibitory T-cell receptor; therapy with an antineoplastic antibody in the previous 4 weeks; other antineoplastic therapy in the previous 2 weeks; or unresolved AEs from previous therapies. The primary end point was the objective response rate (ORR) and the proportion of patients with a complete response (CR) or partial response (PR). DOR was one of the secondary end points.65
Responses to pembrolizumab monotherapy in the KEYNOTE-158 trial are shown in the Figure.65 The ORR among all patients in the cervical cancer cohort was 12%, which included CR of 3%, PR of 9%, and stable disease (SD)of 18%. DOR was not reached.
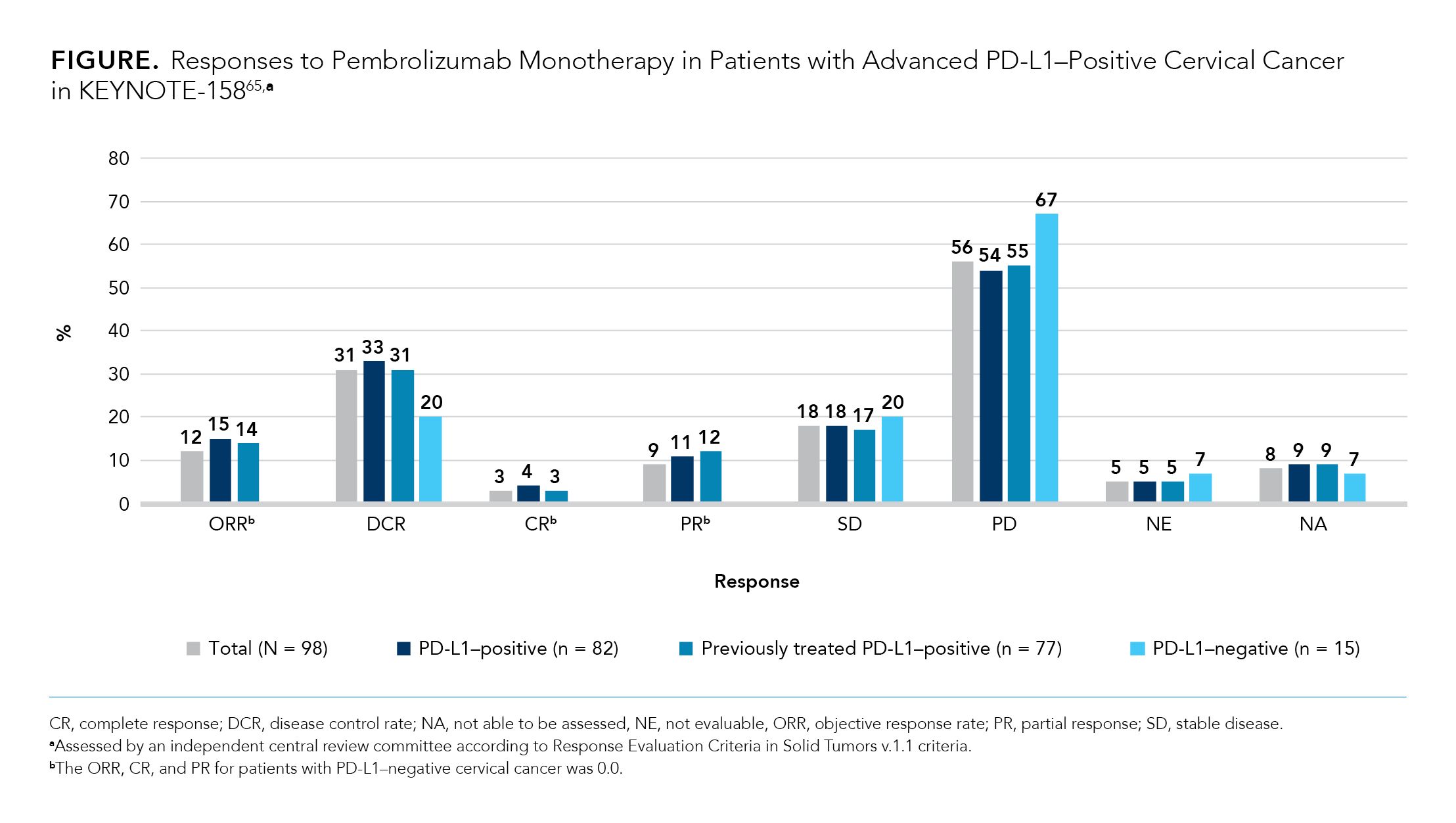
Patients with PD-L1–negative tumors (n = 15) did not obtain CR or PR with pembrolizumab, but 20% obtained SD. Conversely, among patients in the cervical cancer cohort with PD-L1–positive tumors (n = 82) the ORR was 15% (CR, 4%; PR, 11%; SD, 18%); of these, the ORR among patients whose disease was previously treated (n = 77) was 14% (CR, 3%; PR 12%; SD, 17%).65
Disease progressed in 56% of patients in the cervical cancer cohort, which included 54% of patients with PD-L1–positive tumors and 67% of patients with PD-L1–negative tumors.
Among patients in the cervical cancer cohort, no treatment-related mortality was observed, and 4 patients discontinued treatment because of AEs.65
Conclusion
Advanced and recurring cervical cancer is associated with a considerable economic and health care burden. Increased rates of screening and the introduction of the HPV vaccine have led to a decline in the incidence rates of cervical cancer in the past decades.1-4 However, the drastic decrease in routine screening and preventive procedures during the COVID-19 pandemic may increase the risk of late detection of the disease.7 This risk is particularly concerning, because 5-year survival rates for patients with recurrent and advanced disease are drastically lower than for those with early-stage and/or localized cervical cancer.8 Consequently, more treatment options are needed for patients with advanced disease.
Immunotherapies, including PD-1/PD-L1 inhibitors, have shown improved responses in patients with recurring or advanced cervical cancer, supporting the development of these agents to expand therapeutic options.62-65 The next article will discuss these and other therapeutic approaches currently in clinical trials.
References
- Fowler JR, Maani EV, Jack BW. Cervical cancer. In:StatPearls. Updated July 7, 2021. Accessed September 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK431093/?report=classic
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
- Cost-effectiveness of cervical cancer interventions. Centers for Disease Control and Prevention. Updated August 18, 2021. Accessed September 20, 2021.
https://www.cdc.gov/chronicdisease/programs-impact/pop/cervical-cancer.htm - Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. doi:10.1093/jnci/djq495
- Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175-201. doi:10.1093/jnci/djs491
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
- DeGroff A, Miller J, Sharma K, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer Early Detection Program, January-June 2020, in the United States. Prev Med. 2021;151:106559. doi:10.1016/j.ypmed.2021.106559
- Survival rates for cervical cancer. In: Cervical cancer early detection, diagnosis, and staging. American Cancer Society. Updated February 2, 2021. Accessed September 9, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8601.00.pdf
- Cancer facts & figures 2021. American Cancer Society. January 12, 2021. Accessed October 11, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321-346. doi:10.3322/caac.21628
- Biospace. FDA approves Merck & Co., Inc.’s Gardasil, the world’s first and only cervical cancer vaccine. News release. June 8, 2006. Accessed November 23, 2021.https://www.biospace.com/article/releases/fda-approves-merck-and-co-inc-s-gardasil-r-the-world-s-first-and-only-cervical-cancer-vaccine-/
- United States cancer statistics: data visualizations: cancer statistics at a glance. Centers for Disease Control and Prevention. Accessed September 20, 2021. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/
- Key statistics for cervical cancer. In: About cervical cancer. American Cancer Society. Updated January 12, 2021. Accessed September 20, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8599.00.pdf
- Risk factors for cervical cancer. In: Cervical cancer causes, risk factors, and prevention. American Cancer Society. Updated January 3, 2020. Accessed September 20, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8600.00.pdf
- Ault KA. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect Dis Obstet Gynecol. 2006;2006(suppl 2006):40470.doi:10.1155/IDOG/2006/40470
- Cubie HA. Diseases associated with human papillomavirus infection. Virology. 2013;445(1-2):21-34. doi:10.1016/j.virol.2013.06.007
- Wang X, Huang X, Zhang Y. Involvement of human papillomaviruses in cervical cancer. Front Microbiol. 2018;9:2896. doi:10.3389/fmicb.2018.02896
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(suppl 1):S1-S15. doi:10.1016/j.vaccine.2005.09.054
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890-907. doi:10.1016/S0140-6736(07)61416-0
- Plummer M, Herrero R, Franceschi S, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case--control study. Cancer Causes Control. 2003;14(9):805-814. doi:10.1023/b:caco.0000003811.98261.3e
- International Collaboration of Epidemiological Studies of Cervical Cancer, Appleby P, Beral V, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609-1621. doi:10.1016/S0140-6736(07)61684-5
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085-1092. doi:10.1016/S0140-6736(02)08150-3
- Abraham AG, D’Souza G, Jing Y, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62(4):405-413. doi:10.1097/QAI.0b013e31828177d7
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59-67. doi:10.1016/S0140-6736(07)61050-2
- Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365(14):1304-1314. doi:10.1056/NEJMoa1013961
- What is cervical cancer? In: About cervical cancer. American Cancer Society. Updated July 30, 2020. Accessed September 9, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8599.00.pdf
- Cervical cancer treatment (PDQ)-health professional version. National Cancer Institute. Updated January 22, 2021. Accessed September 20, 2021. https://www.cancer.gov/types/cervical/hp/cervical-treatment-pdq#_388
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927-935. doi:10.1002/ijc.25396
- HPV and cancer. National Cancer Institute. Updated October 25, 2021. Accessed October 26, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer
- Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol. 2014;234(4):431-435. doi:10.1002/path.4424
- What causes cervical cancer? In: Cervical cancer causes, risk factors, and prevention. American Cancer Society. Updated January 3, 2020. Accessed September 9, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8600.00.pdf
- Signs and symptoms of cervical cancer. In: Cervical cancer early detection, diagnosis, and staging.American Cancer Society. Updated January 3, 2020. Accessed September 20, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8601.00.pdf
- Tests for cervical cancer. In: Cervical cancer early detection, diagnosis, and staging. American Cancer Society. Updated July 30, 2020. Accessed September 9, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8601.00.pdf
- Cervical cancer stages. In: Cervical cancer early detection, diagnosis, and staging. American Cancer Society. Updated January 3, 2020. Accessed September 9, 2021. https://www.cancer.org/content/dam/CRC/PDF/Public/8601.00.pdf
- Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129-135. doi:10.1002/ijgo.12749
- Stehman FB, Bundy BN, DiSaia PJ, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with radiation therapy. I. A multi-variate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67(11):2776-2785. doi:10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l
- Maiman M, Fruchter RG, Guy L, Cuthill S, Levine P, Serur E. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer. 1993;71(2):402-406. doi:10.1002/1097-0142(19930115)71:2<402::aid-cncr2820710222>3.0.co;2-y
- Bourhis J, Le MG, Barrois M, et al. Prognostic value of c-myc proto-oncogene overexpression in early invasive carcinoma of the cervix. J Clin Oncol. 1990;8(11):1789-1796. doi:10.1200/JCO.1990.8.11.1789
- Burger RA, Monk BJ, Kurosaki T, et al. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J Natl Cancer Inst. 1996;88(19):1361-1368. doi:10.1093/jnci/88.19.1361
- Lai CH, Chang CJ, Huang HJ, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. 2007;25(24):3628-3634. doi:10.1200/JCO.2007.11.2995
- Cervix uteri. SEER 5-year relative survival rates, 2011-2017. SEER Explorer. Updated September 27, 2021. Accessed October 16, 2021. https://seer.cancer.gov/explorer/application.html?site=57&data_type=4&graph_type=5&compareBy=stage&chk_stage_101=101&chk_stage_104=104&chk_stage_105=105&chk_stage_106=106&chk_stage_107=107&series=9&hdn_sex=3&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&advopt_display=1
- Treatment options for cervical cancer by state. American Cancer Society. Updated January 3, 2020. Accessed September 20, 2020. https://www.cancer.org/cancer/cervical-cancer/treating/by-stage.html
- Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154-1161. doi:10.1056/NEJM199904153401503
- Keys HM, Bundy BN, Stehman FB, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003;89(3):343-353. doi:10.1016/s0090-8258(03)00173-2
- Kokka F, Bryant A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. 2015;(4):CD010260. doi:10.1002/14651858.CD010260.pub2
- Small W Jr, Strauss JB, Jhingran A, et al. ACR Appropriateness Criteria definitive therapy for early-stage cervical cancer. Am J Clin Oncol. 2012;35(4):399-405. doi:10.1097/COC.0b013e3182610537
- Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137-1143. doi:10.1056/NEJM199904153401501
- Peters WA III, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606-1613. doi:10.1200/JCO.2000.18.8.1606
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153. doi:10.1056/NEJM199904153401502
- Dubay RA, Rose PG, O’Malley DM, Shalodi AD, Ludin A, Selim MA. Evaluation of concurrent and adjuvant carboplatin with radiation therapy for locally advanced cervical cancer. Gynecol Oncol. 2004;94(1):121-124. doi:10.1016/j.ygyno.2004.03.034
- Chemotherapy for cervical cancer. American Cancer Society. Updated January 3, 2020. Accessed October 28, 2021. https://www.cancer.org/cancer/cervical-cancer/treating/chemotherapy.html
- Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25(20):2952-2965. doi:10.1200/JCO.2007.10.8324
- Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29(13):1678-1685. doi:10.1200/JCO.2009.25.9663
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802-5812. doi:10.1200/JCO.2008.16.4368
- Avastin. Prescribing information. Genentech; 2021. Accessed October 28, 2021. https://www.avastin.com/hcp.html
- Keytruda. Prescribing information. Merck Sharp & Dohme Corp; 2021. Accessed October 28, 2021. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- Thigpen JT, Blessing JA, DiSaia PJ, Fowler WC Jr, Hatch KD. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1989;32(2):198-202. doi:10.1016/s0090-8258(89)80033-2
- Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 1999;17(9):2676-2680. doi:10.1200/JCO.1999.17.9.2676
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306-1309. doi:10.1126/science.2479986
- Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671-680.
- Tewari KS, Sill MW, Long HJ III, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734-743. doi:10.1056/NEJMoa1309748
- FDA approves pembrolizumab for advanced cervical cancer with disease progression during or after chemotherapy. News release. FDA. June 18, 2018. Accessed September 23, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-cervical-cancer-disease-progression-during-or-after-chemotherapy
- Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212-1218. doi:10.1038/ni.2762
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293-12297. doi:10.1073/pnas.192461099
- Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470-1478. doi:10.1200/JCO.18.01265.

