- Center on Health Equity & Access
- Clinical
- Health Care Cost
- Health Care Delivery
- Insurance
- Policy
- Technology
- Value-Based Care
A Concise Review of the Changing Landscape of Hepatocellular Carcinoma
Abstract
Hepatocellular carcinoma (HCC) is the fastest rising cause of cancer-related deaths in the United States, increasing by 2% to 3% annually, with a dismal 5-year survival rate of 18%. The Barcelona Clinic Liver Cancer criteria used to guide treatment considers performance status and assessment of liver function by Child-Pugh score in addition to tumor size and location. Curative therapies for HCC include surgical resection, liver transplantation, and tumor ablation. Patients with unresectable or inoperable lesions should be considered for arterially directed embolic therapy, systemic therapy, or radiation. Options for first-line systemic therapy of advanced HCC include sorafenib, lenvatinib, and atezolizumab plus bevacizumab. Nivolumab may be an option in patients with advanced HCC who are ineligible for tyrosine kinase inhibitors or other anti-angiogenic agents. Options for subsequent therapy following disease progression include regorafenib, cabozantinib, ramucirumab, lenvatinib, nivolumab, nivolumab plus ipilimumab, sorafenib, or pembrolizumab. Patients with advanced HCC are at a high risk of adverse effects because of baseline hepatic dysfunction, comorbidities associated with chronic liver disease, and potential drug−drug interactions. Improved tolerance of therapies for advanced HCC may lead to reduction in treatment discontinuation and contribute to better patient outcomes. Managed care pharmacists should understand the recent efficacy and safety data, guideline recommendations, and treatment algorithms for management of HCC.
Am J Manag Care. 2020;26:S211-S219. https://doi.org/10.37765/ajmc.2020.88512
Introduction
The American Cancer Society estimates nearly 43,000 patients will be diagnosed with liver and intrahepatic bile duct cancers in 2020, with an estimated 30,160 deaths.1 Hepatocellular carcinoma (HCC) has been the fastest rising cause of cancer-related deaths in the United States, increasing by 2% to 3% annually.1 The majority (71%) of cases of HCC are potentially preventable. The main risk factors for HCC include alcohol, tobacco, nonalcoholic fatty liver disease, and hepatitis B and C viruses, and an increase in incidence is anticipated due to the rise in hepatitis C infection corresponding to the opioid epidemic.1,2 Sustained inflammation, hepatocyte necrosis and regeneration, and fibrotic deposition lead to development of cirrhosis.3 Over time, the accumulation of somatic genomic alterations in genes and epigenetic modifications in hepatocytes favors development of HCC.4
Diagnosis and Staging
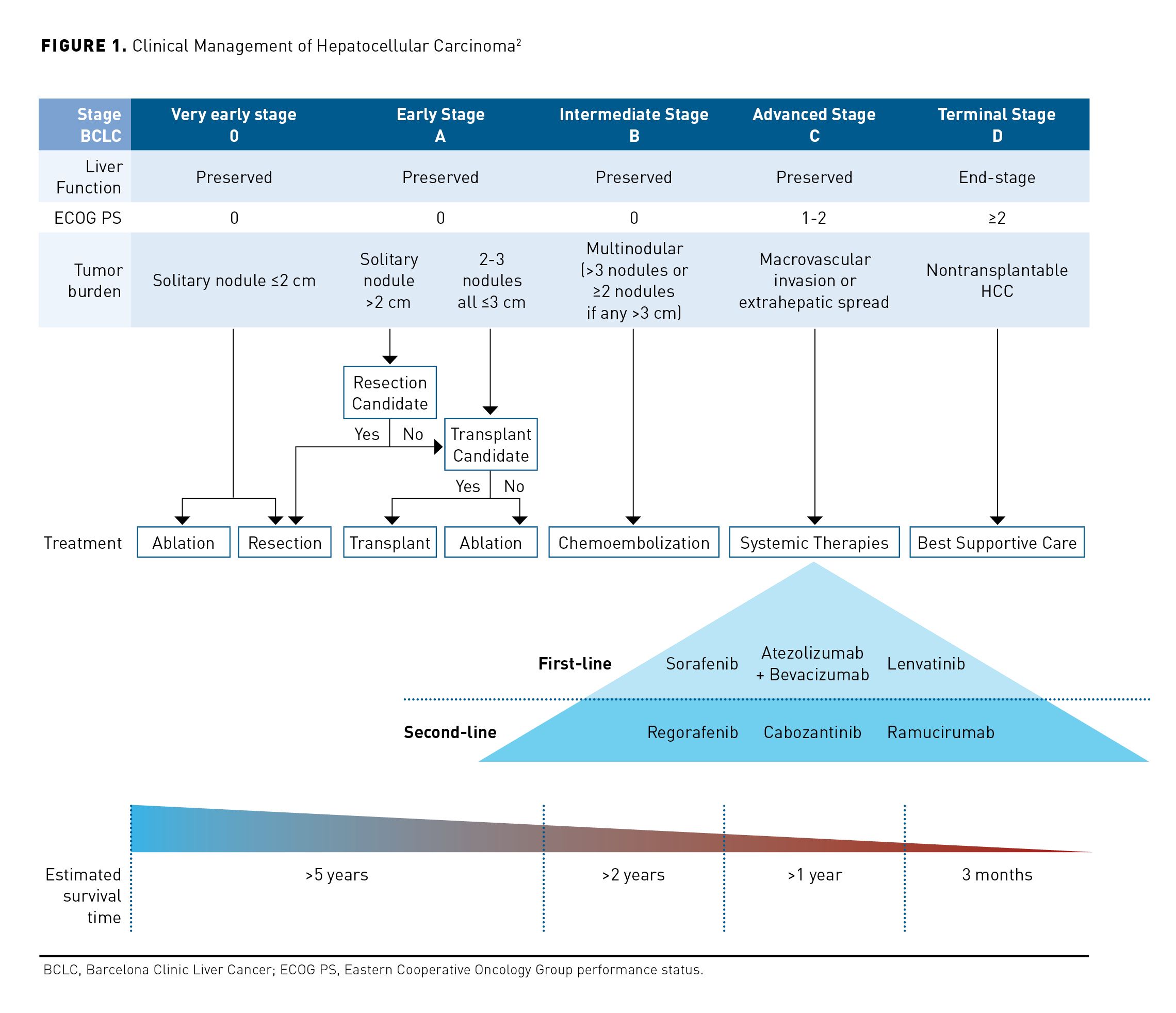
Patients with early-stage HCC are often asymptomatic and because symptoms are vague, most patients present with advanced disease at diagnosis. Symptoms may include loss of appetite, weight loss, early satiety, nausea or vomiting, hepatomegaly, splenomegaly, abdominal or right shoulder blade pain, ascites, pruritus, and jaundice.3 Surveillance testing with ultrasound every 6 months is cost-effective in patients with cirrhosis and chronic hepatitis B and should be offered to these patients to facilitate early detection of HCC and improve overall survival (OS).2 The American Association for the Study of Liver Diseases recommends surveillance with ultrasound and alfa-fetoprotein (AFP) every 6 months for patients with cirrhosis and a high risk of HCC.2 Unlike most solid tumors where a biopsy is needed for diagnosis, the diagnosis of HCC is based on interpretation of multiphase computed tomography (CT) scan or magnetic resonance imaging (MRI) in patients with cirrhosis.2 The 2 commonly used systems for staging HCC are the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system, which just characterizes tumor features and metastases, and the Barcelona Clinic Liver Cancer (BCLC) staging system, which combines tumor characteristics with assessment of severity of liver disease and functional status of the patient.5,6 The TNM system has been validated for both hepatic resection and transplant, while the BCLC staging system is recommended for nonsurgical patients with advanced HCC, as it offers prognostic information based on assessment of tumor burden, liver function, and patient performance status (PS) (Figure 1).2 For treatment stratification, HCC disease status is classified as resectable, transplantable, unresectable, or metastatic.7
AFP is considered positive if its value is >20ng/mL and negative if lower.2 A positive or rising AFP should prompt CT scan or MRI regardless of ultrasound results.7
Treatment
Multidisciplinary management, including hepatologists, oncologists, surgeons, and specialized pharmacists and nurses, is critical to management of patients with advanced HCC because most patients have concomitant liver disease.3 The potential harms and benefits of therapy must be balanced. Accurate assessment of PS, generally with the Eastern Cooperative Oncology Group (ECOG) status, is essential to the management of HCC, as it is used to guide treatment in all phases of disease.8 Patients with advanced HCC are at high risk for adverse effects (AEs) because of baseline hepatic dysfunction, comorbidities associated with chronic liver disease (eg, cytopenias, diarrhea, fatigue, hypothyroidism, hepatorenal syndrome, esophageal varices, and cardiomyopathy), and potential drug−drug interactions (DDIs) (eg, immunosuppressants, HIV or hepatitis C therapies, and cardiovascular agents).9,10
Curative Therapies
Patients with a solitary tumor, good PS, BCLC stage 0 or A disease with preserved liver function and no significant portal hypertension are ideal candidates for surgical resection.2,3 Surgical resection is associated with a 5-year survival over 60%; however, 70% of these patients will have recurrence within 5 years.3 Liver transplantation is offered as a curative therapy to a select group of patients with limited tumor burden who are not candidates for resection.3 Transplantation of this subgroup of patients with HCC is associated with a 5-year overall survival (OS) of 60% to 80% with posttransplant recurrence in fewer than 15% of patients. Tumor ablation is recommended for patients with BCLC stage 0 or A disease who are not candidates for surgery.3 Stereotactic body radiation is an alternative to ablation.2
Palliative Therapies
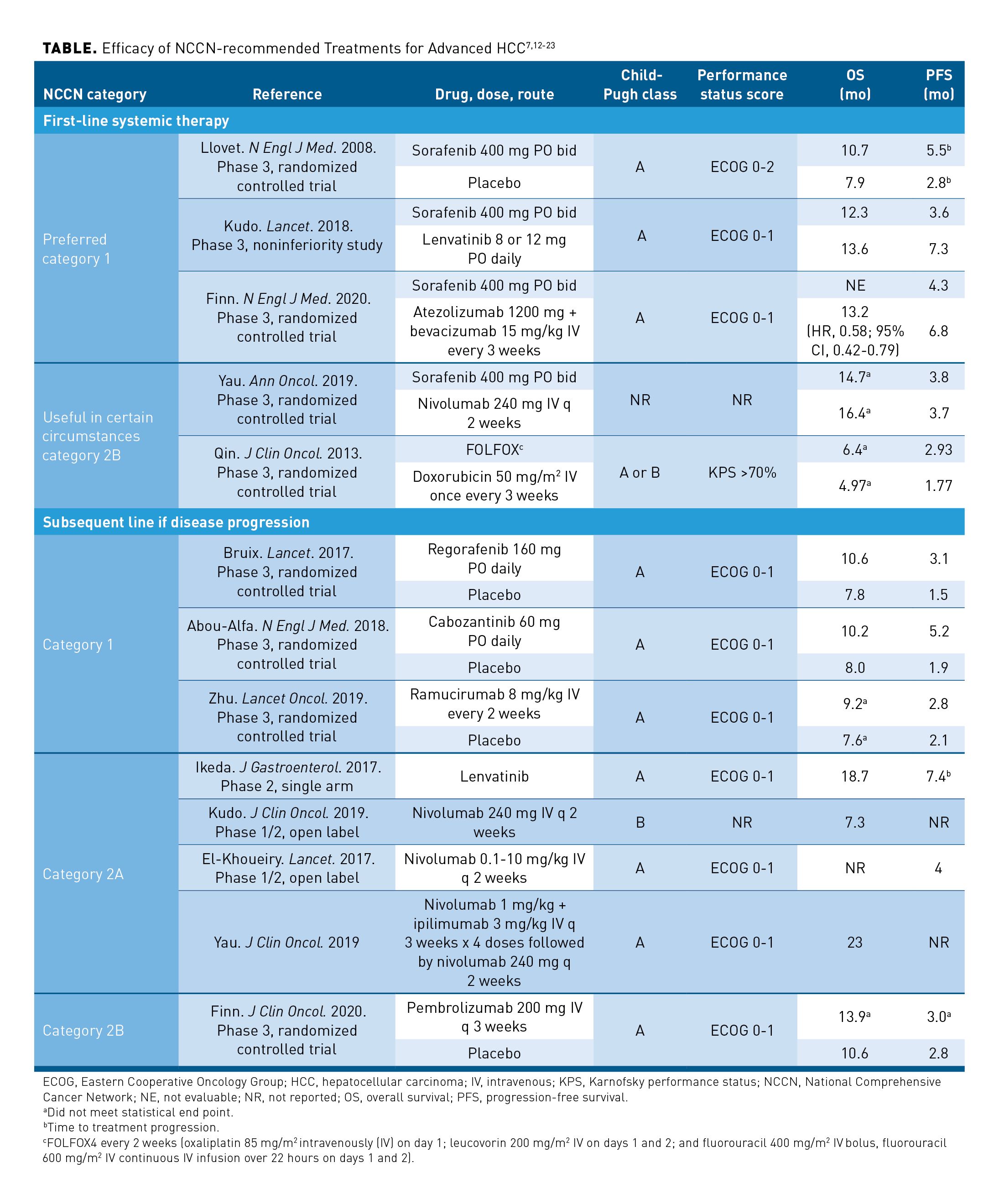
Patients with unresectable or inoperable lesions more than 5 cm in diameter should be considered for arterially directed therapy, systemic therapy, or external beam radiation.7 Transarterial chemoembolization (TACE) is the preferred treatment approach for patients with BCLC stage B tumors with compensated cirrhosis, involving infusion of cytotoxic chemotherapy into a hepatic artery, followed immediately by embolization of the blood vessels that feed the tumor. TACE is associated with an objective response rate of 52.5% with a median survival of 20 to 26 months.11 Transarterial radioembolization (TARE) refers to the infusion of microspheres of a radioisotope, yttrium-90, into the hepatic artery, and is also appropriate for patients with BCLC stage B tumors. No randomized studies have compared TARE with TACE with respect to survival; however, cohort and retrospective studies indicate the objective response rates and OS are similar.2 For patients with unresectable disease, poor liver function, or metastatic disease, systemic targeted therapy is recommended in addition to locoregional therapy modalities.7 Options for first-line systemic therapy of advanced HCC include sorafenib, lenvatinib, and atezolizumab plus bevacizumab (Table7,12-23). Nivolumab may be useful in patients with advanced HCC who are ineligible for tyrosine kinase inhibitors (TKIs) or other anti-angiogenic agents. Limited data support the use of fluorouracil, leucovorin, oxaliplatin (FOLFOX); therefore, enrollment in a clinical trial is recommended for patients receiving cytotoxic chemotherapy.7 Options for subsequent therapy following disease progression include regorafenib, cabozantinib, ramucirumab, lenvatinib, nivolumab, nivolumab plus ipilimumab, sorafenib, or pembrolizumab.7 Larotrectinib and entrectinib are treatment options for patients with HCC who are NTRK gene fusion positive.7
Angiogenesis Inhibition
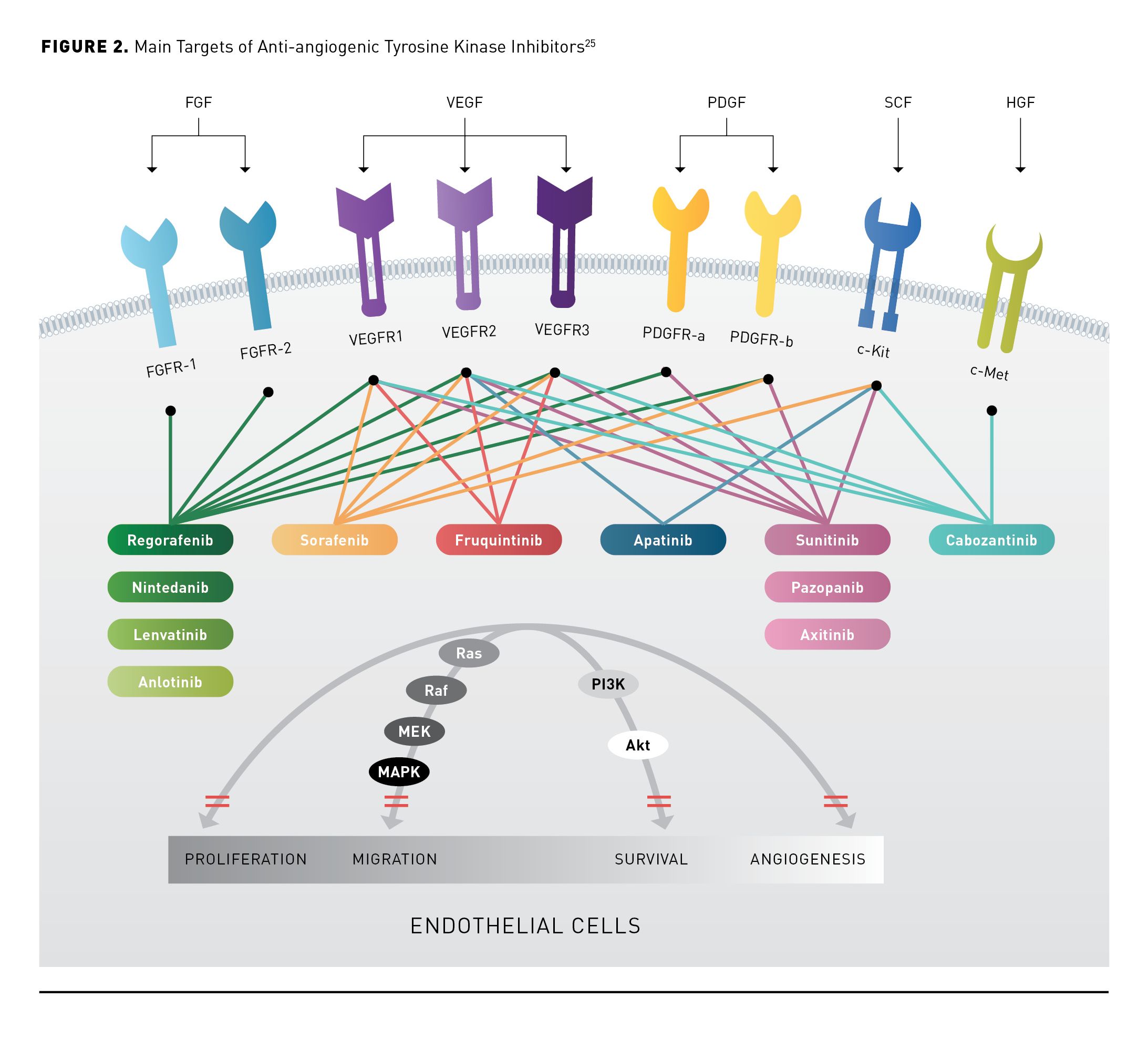
Based on the theory that tumor growth, progression, and metastasis depend on angiogenesis and the hypervascular nature of most HCC tumors, multiple TKIs and monoclonal antibodies (mAbs) have been developed to target angiogenesis inhibition.24 Several angiogenic pathways are dysregulated in HCC, suggesting they may be involved in the development and pathogenesis of HCC. Circulating vascular endothelial growth factor (VEGF) levels are increased in HCC and have been shown to correlate with tumor angiogenesis and progression.24 The anti-angiogenic TKIs target VEGF or the VEGF receptor (VEGFR) (Figure 225). Monoclonal antibodies block VEGF from interacting with receptors, ultimately leading to angiogenesis inhibition. Bevacizumab binds VEGF and ramucirumab binds VEGFR2.26,27
VEGF TKIs
Comorbidities, such as impaired liver function and genetic variations in liver enzyme function, should be considered when dosing TKIs or determining the etiology of AEs.9 TKIs are associated with AEs that may negatively impact patients’ health-related quality of life.9 The incidence of grade 3 or higher AEs ranges from 45% to 75% in phase 3 clinical trials with sorafenib, regorafenib, lenvatinib, and cabozantinib in patients with HCC.9 The most frequently observed AEs, including fatigue, diarrhea, hand-foot skin reaction, nausea, vomiting, anorexia, weight loss, and hypertension, are similar for these agents. AEs associated with TKIs commonly lead to dose interruptions, dose reductions, and treatment discontinuation in HCC.9 If TKI tolerability can be improved, patients are more likely to remain on treatment and therefore may experience better treatment outcomes.28-30
In general, monitoring parameters are similar among TKIs used to manage HCC. Patients should have liver function tests (LFTs) monitored routinely. Baseline monitoring for proteinuria and an electrocardiogram for QTc prolongation should be completed before initiation of therapy and repeated as clinically indicated. Thyroid-stimulating hormone should be assessed at baseline and monthly thereafter. Other potential AEs with VEGF TKIs include cardiac ischemia, arterial thromboembolism, congestive heart failure, bleeding, reversible posterior leukoencephalopathy syndrome, gastrointestinal perforation, and impaired wound healing, all of which require treatment interruption and/or discontinuation.31-34
The TKIs sorafenib, regorafenib, lenvatinib, and cabozantinib are metabolized by the liver enzyme CYP3A4 and concomitant use with CYP3A4 inducers or inhibitors may increase toxicity or decrease TKI efficacy.9 Strong CYP3A4 inhibitors should be avoided with regorafenib and cabozantinib but may be used with sorafenib and lenvatinib. Inducers of CYP3A4 should be avoided with sorafenib, regorafenib, and cabozantinib; however, they may be used with lenvatinib.9 Only sorafenib and nivolumab have been shown to be safe in patients with Child-Pugh class B disease.12,35-37
VEGF mAbs
DDIs are typically not observed with mAbs. AEs with ramucirumab and bevacizumab are consistent with those observed with VEGF inhibitors. Grade 3 or greater AEs occurring in 5% or more of patients receiving ramucirumab for second-line therapy of HCC include hypertension (12%), as well as ascites, asthenia, increased aspartate aminotransferase (AST), and thrombocytopenia (all 5%); 10% of patients discontinued ramucirumab due to AEs.13 The most common treatment-related AEs grade 3 or greater observed in more than 5% of patients receiving atezolizumab plus bevacizumab patients were hypertension (15.2%) and increased AST (5%).14 Treatment-related AEs leading to discontinuation were observed in 15.5% of patients treated with atezolizumab plus bevacizumab. Possible serious AEs associated with VEGF mAbs include bleeding, gastrointestinal perforation or fistula development, impaired wound healing, blood clots, proteinuria, hypertension, and infusion-related reactions.26,27
Immune Checkpoint Inhibition
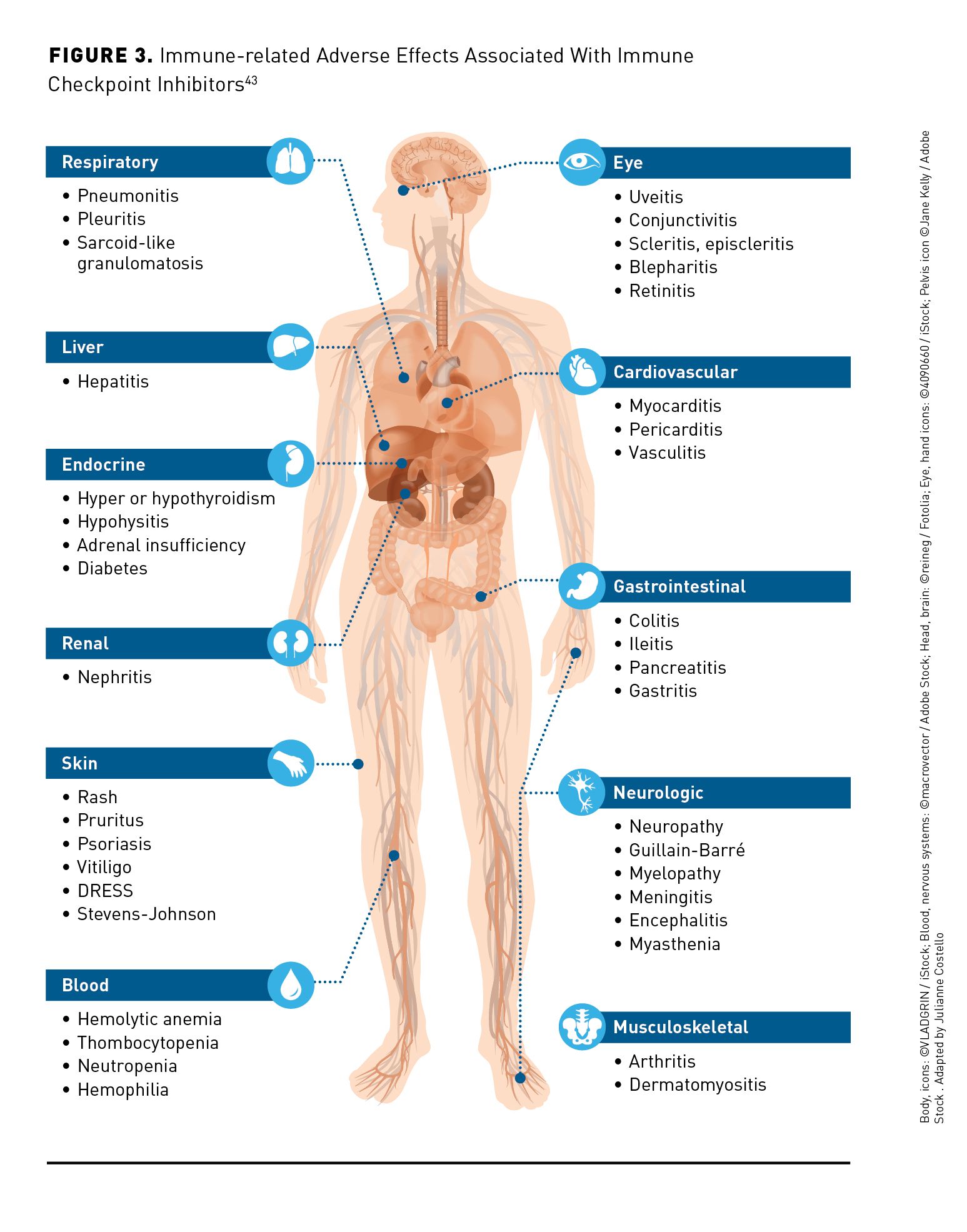
Immune checkpoint inhibitors (ICIs) are mAbs targeting cytotoxic T lymphocyte-associated (CTLA)-4, programmed death (PD)-1, or programmed death ligand-1 (PD-L1). By blocking the negative signals of CTLA-4, PD-1, and PD-L1, ICIs enhance T- cell activation.10 Atezolizumab and durvalumab bind to PD-L1, whereas pembrolizumab and nivolumab bind to the PD-1 receptor.38-41 Monoclonal antibodies that disrupt PD-1 immune checkpoint signaling restore the antitumor activity of otherwise suppressed effector T cells.15 Ipilimumab and the investigational agent tremelimumab are anti-CTLA-4 antibodies.42 Blockade of inhibitory checkpoint molecules results in disruption of immune homeostasis and may result in autoimmune complications. A broad range of immune-related adverse effects (irAEs) involve almost every organ but mostly affect the skin, digestive system, lung, endocrine glands, nervous system, kidney, blood cells, and musculoskeletal system (Figure 343). ICIs are generally better tolerated than TKIs, although some patients experience serious or fatal irAEs.44 In general, the incidence of most irAEs in patients with HCC receiving ICIs is similar to other tumor types, although there is a trend toward a higher incidence of hepatic irAEs.10 The incidence of irAEs is 27% for all grades and 6% for grade 3 or higher with PD-1 or PD-L1 inhibitors. With CTLA-4 inhibitors, the overall incidence of irAEs is higher and fluctuates according to the dose; it may be as high as 72% for all grades and 24% for grade 3 or higher.10
The clinical significance and effect on patient safety and survival of irAEs is variable based on the organ involved, severity of toxicity, and response to therapy.10 Early recognition and treatment of irAEs may be critical to maintaining patients on therapy and delivering successful outcomes. Management of irAEs in HCC is complicated by comorbidities associated with chronic liver disease that may overlap with irAEs and contribute to misdiagnosis or delays in diagnosis of irAEs.10 Management of hepatic irAEs in patients with HCC must also consider baseline liver function tests (LFTs), the potential for spontaneous LFT fluctuations, tumor progression in the liver, viral hepatitis, drug toxicity, and other causes.10 Late recognition of an irAE may delay treatment and worsen prognosis, whereas inappropriate diagnosis of an irAE may lead to interruption of therapy, further complications due to immunosuppressive therapy, the potential for unnecessary interventions, and increased cost.10 The National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) have published guidelines on management of irAEs.45,46 Management of irAEs is based on severity of symptoms and may involve holding ICI therapy, initiation of high-dose corticosteroids or other immunosuppressive therapy, and supportive care.10,45,46
NCCN Guideline Recommendations
First-line Therapy
Sorafenib and lenvatinib are first-line monotherapy options for patients with Child-Pugh class A, unresectable HCC with a category 1 recommendation in the NCCN guidelines (Table).7,12-23 Sorafenib is the current standard of care based on a phase 3 trial demonstrating superiority over placebo.16 Results of a phase 3 study demonstrated lenvatinib is noninferior to sorafenib in terms of OS and superior in terms of progression-free survival (PFS), time to tumor progression (TTP), and overall response rate (ORR).17 Quality of life declined in both groups after treatment, although the decline occurred more slowly in some domains for patients treated with lenvatinib. Based on these data, lenvatinib was approved by the FDA for the first-line treatment of unresectable HCC.32
ICIs are a first-line option for advanced HCC based on the randomized, phase 3, IMbrave150 trial comparing atezolizumab 1200 mg IV plus bevacizumab 15 mg/kg IV every 3 weeks with sorafenib 400 mg orally twice daily.14 Hypoxia stimulates tumor angiogenesis by upregulation of hypoxia-inducible factor proteins, which induce expression of proangiogenic factors, including VEGF.24 Alleviation of tumor hypoxia via VEGF inhibition is hypothesized to reduce VEGF-mediated immunosuppression within the tumor and its microenvironment, ultimately enhancing anti−PD-1 and anti−PD-L1 efficacy by reversing VEGF-mediated immunosuppression and promoting T-cell tumor infiltration.47 The IMbrave150 study demonstrated that combination therapy with atezolizumab showed an improvement in PFS (6.8 months vs 4.3 months) and OS (HR, 0.58; 95% CI, 0.42-0.79; P <.001) with a similar risk of grade 3 or 4 AEs (56.5% vs 55.1%) compared with sorafenib monotherapy. Atezolizumab in combination with bevacizumab is FDA approved for unresectable or metastatic HCC in patients who have not received prior systemic therapy and is a category 1 recommendation in the NCCN guidelines.7,40
Nivolumab may be considered as first-line monotherapy for patients ineligible for TKIs or other anti-angiogenic agents.7 Nivolumab failed to demonstrate superiority in a phase 3 comparison with sorafenib.18 The NCCN guidelines recommend use of chemotherapy in the context of a clinical trial.7 FOLFOX failed to demonstrate superior OS in a phase 3 comparison with doxorubicin.19
Subsequent Therapy
Regorafenib, cabozantinib, and ramucirumab are all FDA approved and have category 1 recommendations in the NCCN guidelines for patients with HCC with disease progression on first-line therapy (Table).7,12-23 Ramucirumab use is limited to patients with an AFP greater than or equal to 400 ng/mL.26 All 3 agents demonstrated improvements in PFS and OS compared with placebo in patients with progressive disease on sorafenib in phase 3 clinical trials.13,20,21 A phase 2, single-arm study of lenvatinib reported a median OS of 18.7 months and TTP of 7.4 months. Just 13% of the patients in this study had received prior therapy with sorafenib and approximately 65% of patients were treatment naïve.22 Lenvatinib and sorafenib are not FDA approved for use in the second-line setting; however, both are endorsed by the NCCN guidelines for use in this setting.7 There are no studies of sorafenib in the second-line setting and the value is unclear in patients who received prior therapy with TKIs or ICIs.
CheckMate-040 is a phase 1/2, open-label clinical trial with multiple cohorts reporting outcomes for patients with advanced HCC receiving nivolumab; approximately two-thirds of patients received prior sorafenib.15 The median PFS of all patients across multiple dose levels was 4 months, with the median OS not reached.15 A higher objective response rate was observed in patients with PD-L1 expression greater than 1%. In patients with Child-Pugh B liver function, the median OS was 7.3 months; approximately half of these patients received prior sorafenib.12 Preliminary results of the cohort receiving nivolumab in combination with ipilimumab demonstrated an ORR of 33% with a response duration ranging from 4.6 to 30.5 months, with 31% of patients having a response duration more than 24 months; all patients received prior sorafenib.39 The most common AEs (≥20%) with the combination of nivolumab and ipilimumab were rash, pruritus, musculoskeletal pain, diarrhea, cough, decreased appetite, fatigue, pyrexia, abdominal pain, dyspnea, headache, nausea, hypothyroidism, decreased weight, and dizziness in contrast with fatigue, musculoskeletal pain, abdominal pain, diarrhea, pruritus, rash, cough, and decreased appetite with monotherapy. Nivolumab (240 mg every 2 weeks or 480 mg every 4 weeks) is approved by the FDA as a single agent or in combination (nivolumab 1 mg/kg followed by ipilimumab 3 mg/kg intravenous on the same day every 3 weeks for 4 doses followed by nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks) in patients with HCC who have been previously treated with sorafenib.39
Pembrolizumab was recently approved by the FDA for the treatment of patients with HCC who have been previously treated with sorafenib.38 The most recent version of the NCCN guidelines endorses pembrolizumab in patients with Child-Pugh A disease as a category 2B recommendation. A phase 3, randomized controlled trial comparing pembrolizumab to placebo did not meet the statistical end point for superiority of PFS or OS; a median OS of 13.9 months and PFS of 3.0 months was reported in patients receiving pembrolizumab.23
Investigational Therapies
ICI Monotherapy
Nivolumab is currently being evaluated in the CheckMate 459 phase 3 clinical trial in the first-line setting compared with sorafenib.18 Preliminary results demonstrated a median OS of 16.4 months for nivolumab compared with 14.7 months for sorafenib (P = .04); this did not meet the predefined threshold of statistical significance.18
Combination Therapy With ICIs
Phase 2 data were presented at the 2020 ASCO Annual Meeting for Study 22, a trial comparing tremelimumab 300 mg x 1 dose or 75 mg x 4 doses in combination with durvalumab 1500 mg every 4 weeks, with durvalumab 1500 mg every 4-week monotherapy, or tremelimumab 750 mg every 4 weeks for 7 doses followed by every 12 weeks thereafter. A median OS of 18.73 months and an ORR of 24% were seen with tremelimumab 300 mg x 1 dose combined with durvalumab 1500 mg q 4 weeks; the median duration of survival had not been reached.42 Results of the phase 3 HIMALAYA trial comparing durvalumab plus tremelimumab combination therapy to durvalumab monotherapy or sorafenib monotherapy in the treatment of patients with no prior systemic therapy for unresectable HCC in HCC are expected in late 2020.48
Another trial of combination therapy is the phase 1b Study 116/KEYNOTE-524 of pembrolizumab 200 mg IV every 3 weeks and lenvatinib daily (8 mg for patients less than or equal to 60 kg or 12 mg for patients over 60 kg).49 Results presented at the 2020 ASCO Annual Meeting reported an ORR of 36% and a median duration of response of 12.6 months. The study is ongoing; however, the FDA declined to provide accelerated approval of the combination on July 8, 2020.50
Vaccination
Cancer vaccines are designed to promote tumor-specific immune responses and increase specific cytotoxic CD8-positive T cells.51 In phase 1 or 2 clinical trials, AFP, glypican-3, multidrug resistance-associated protein 3, cancer-testis antigen, and human telomerase reverse transcriptase vaccines were safe and well tolerated.51,52 Future research for vaccines is needed to identify more HCC-specific tumor-associated antigens and improve the response to vaccine therapies.52
Conclusions
Multiple options are now available for treatment of advanced HCC in both the first- and second-line settings. There is no preferred sequence of therapy and treatment decisions may be based on patient factors, tolerance to prior therapies, cost, and patient preferences. Patients with advanced HCC are a high-risk population for AEs and polypharmacy because of baseline hepatic dysfunction, comorbidities associated with chronic liver disease, and potential DDIs.10 Since 2017, the FDA has approved 9 medications for the treatment of advanced HCC.2 Given the number of new medications entering the market and phase 3 clinical trial data available, managed care professionals should have an understanding of the clinical trial data, guideline recommendations, and treatment algorithms for management of HCC.
Author affiliation: Amber Draper, PharmD, BCOP, is a clinical pharmacy specialist in GI oncology and a clinical coordinator in oncology, Emory Winship Cancer Institute, Atlanta, GA.
Funding source: This activity is supported by an educational grant from Genentech, a member of the Roche Group.
Author disclosure: Dr Draper has no relevant financial relationships with commercial interests to disclose.
Authorship information: Drafting of the manuscript and critical revision of the manuscript for intellectual content.
Address correspondence to: amber.draper@emoryhealthcare.org.
Medical writing and editorial support provided by: Julianna Merten, PharmD, BCPS, BCOP.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi: 10.1002/hep.29913
3. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462. doi: 10.1056/NEJMra1713263
4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301-1314. doi: 10.1016/s0140-6736(18)30010-2
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329-338. doi: 10.1055/s-2007-1007122
6. Amin MB, Edge S, Greene F, et al (eds.). AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017 [cited December 28, 2016].
7. Hepatocellular carncinoma. National Comprehensive Cancer Network Guideliines version 4.2020. Updated May 19, 2020. Accessed July 2, 2020. nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
8. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
9. Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20-28. doi: 10.1016/j.ctrv.2019.05.004
10. Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72(2):320-341. doi: 10.1016/j.jhep.2019.10.021
11. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106-116. doi: 10.1002/hep.28453
12. Kudo M, Matilla A, Santoro A, et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J Clin Oncol. 2019;37(4_suppl):327. doi: 10.1200/JCO.2019.37.4_suppl.327
13. Zhu AX, Park JO, Ryoo B-Y, et al; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859-870. doi: 10.1016/s1470-2045(15)00050-9
14. Finn RS, Qin S, Ikeda M, et al; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi: 10.1056/NEJMoa1915745
15. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/s0140-6736(17)31046-2
16. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi: 10.1056/NEJMoa0708857
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi: 10.1016/S0140-6736(18)30207-1
18. Yau T, Park JW, Finn RS, et al. CHECKMATE 459: a randomized, multi-center phase 3 study of nivolumab vs sorafeniib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol. 2019;30(suppl 5):v851-v934. doi: 10.1093/annonc/mdz394
19. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501-3508. doi: 10.1200/jco.2012.44.5643
20. Bruix J, Qin S, Merle P, et al; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66. doi: 10.1016/s0140-6736(16)32453-9
21. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54-63. doi: 10.1056/NEJMoa1717002
22. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512-519. doi: 10.1007/s00535-016-1263-4
23. Finn RS, Ryoo BY, Merle P, et al; KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193-202. doi: 10.1200/jco.19.01307
24. Morse MA, Sun W, Kim R, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912-920. doi: 10.1158/1078-0432.ccr-18-1254
25.Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12(1):27. doi: 10.1186/s13045-019-0718-5
26. Cyramza. Prescribing information. Eli Lilly and Company; June 2020. Accessed July 3, 2020. pi.lilly.com/us/cyramza-pi.pdf
27. Avastin. Prescribing information. Genentech, Inc; May 2020. Accessed July 3, 2020. gene.com/download/pdf/avastin_prescribing.pdf
28. Brunot A, Le Roy F, Le Sourd S, et al. Implementation of a nurse-driven educational program improves management of sorafenib’s toxicities in hepatocellular carcinoma. Cancer Nurs. 2018;41(5):418-423. doi: 10.1097/ncc.0000000000000521
29. Ponziani FR, Bhoori S, Germini A, et al. Inducing tolerability of adverse events increases sorafenib exposure and optimizes patient’s outcome in advanced hepatocellular carcinoma. Liver Int. 2016;36(7):1033-1042. doi: 10.1111/liv.13052
30. Scandurra G, Aiello RA, Ali M, et al. Appropriate management of cutaneous adverse events maximizes compliance with sorafenib treatment: a single-center experience. Future Oncol. 2012;8(5):609-615. doi: 10.2217/fon.12.35
31. Cabometyx. Prescribing information. Exelixis, Inc; July 2020. Accessed August 30, 2020. cabometyxhcp.com/pi/
32. Lenvima. Prescribing information. Eisai Inc; July 2020. Accessed August 30, 2020. lenvima.com/pdfs/prescribing-information.pdf
33. Stivarga. Prescribing information. Bayer HealthCare Pharmaceuticals Inc; June 2020. Accessed July 3, 2020. labeling.bayerhealthcare.com/html/products/pi/Stivarga_PI.pdf
34. Nexavar. Prescribing information. Bayer HealthCare Pharmaceuticals Inc; July 2020. Accessed August 30, 2020. labeling.bayerhealthcare.com/html/products/pi/Nexavar_PI.pdf
35. Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(26):4293-4300. doi: 10.1200/jco.2005.01.3441
36. Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27(11):1800-1805. doi: 10.1200/JCO.2008.20.0931
37. Kambhampati S, Bauer KE, Bracci PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019;125(18):3234-3241. doi: 10.1002/cncr.32206
38. Keytruda. Prescribing information. Merck Sharp & Dohme Corp; June 2020. Accessed July 3, 2020. merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
39. Opdivo. Prescribing information. Bristol Myers Squibb; June 2020. Accessed July 3, 2020. packageinserts.bms.com/pi/pi_opdivo.pdf
40. Tecentriq. Prescribing information. Genentech; July 2020. Accessed August 30, 2020. gene.com/download/pdf/tecentriq_prescribing.pdf
41. Imfinzi. Prescribing information. AstraZeneca; June 2020. Accessed July 3, 2020. azpicentral.com/imfinzi/imfinzi.pdf#page=1
42. Imfinzi plus tremelimumab demonstrated promising clinical activity and tolerability in patients with advanced liver cancer. AstraZeneca. News release. May 29, 2020. Accessed July 2, 2020. astrazeneca.com/media-centre/press-releases/2020/imfinzi-plus-tremelimumab-demonstrated-promising-clinical-activity-and-tolerability-in-patients-with-advanced-liver-cancer.html
43.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559-574. doi: 10.1093/annonc/mdv623
44. Giannini E, Aglitti A, Borzio M, et al; Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI.CA Cohort derived estimate of amenability rate to immune checkpoint inhibitors in clinical practice. Cancers (Basel). 2019;11(11):1689. doi: 10.3390/cancers11111689
45. Management of immunotherapy-related toxicities. National Comprehensive Cancer Network Guidelines version 1.2020. Updated December 16, 2019. Accessed May 24, 2020. nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
46. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi: 10.1200/jco.2017.77.6385
47. Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: looking outside the box. J Hepatol. 2020;72(2):342-352. doi: 10.1016/j.jhep.2019.09.010
48. Study of Durvalumab and Tremelimumab as First-line Treatment in Patients With Advanced Hepatocellular Carcinoma (HIMALAYA). Updated August 27, 2020. Accessed August 29, 2020. clinicaltrials.gov/ct2/show/NCT03298451]
49. Results from Lenvima (lenvatinib) plus Keytruda (pembrolizumab) trials in unresectable hepatocellular carcinoma and advanced renal cell carcinoma to be presented at 2020 ASCO Annual Meeting. Eisai Global. News release. May 29, 2020. Accessed July 1, 2020. eisai.com/news/2020/news202023.html
50. Merck and Eisai receive Complete Response Letter for Keytruda (pembrolizumab) plus Lenvima (lenvatinib) combination as first-line treatment for unresectable hepatocellular carcinoma. News release. Merck and Eisai. July 8, 2020. Accessed July 26, 2020. bwnews.pr/2Z89nmX
51. Sun Z, Zhu Y, Xia J, Sawakami T, Kokudo N, Zhang N. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. BioSci Trends. 2016;10(2):85-91. doi: 10.5582/bst.2015.01128
52. Buonaguro L, HEPAVAC Consortium. New vaccination strategies in liver cancer. Cytokine Growth Factor Rev. 2017;36:125-129. doi: 10.1016/j.cytogfr.2017.06.010
